Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation - PubMed (original) (raw)
Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation
Takafumi Wataya et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Embryonic stem (ES) cells differentiate into neuroectodermal progenitors when cultured as floating aggregates in serum-free conditions. Here, we show that strict removal of exogenous patterning factors during early differentiation steps induces efficient generation of rostral hypothalamic-like progenitors (Rax(+)/Six3(+)/Vax1(+)) in mouse ES cell-derived neuroectodermal cells. The use of growth factor-free chemically defined medium is critical and even the presence of exogenous insulin, which is commonly used in cell culture, strongly inhibits the differentiation via the Akt-dependent pathway. The ES cell-derived Rax(+) progenitors generate Otp(+)/Brn2(+) neuronal precursors (characteristic of rostral-dorsal hypothalamic neurons) and subsequently magnocellular vasopressinergic neurons that efficiently release the hormone upon stimulation. Differentiation markers of rostral-ventral hypothalamic precursors and neurons are induced from ES cell-derived Rax(+) progenitors by treatment with Shh. Thus, in the absence of exogenous growth factors in medium, the ES cell-derived neuroectodermal cells spontaneously differentiate into rostral (particularly rostral-dorsal) hypothalamic-like progenitors, which generate characteristic hypothalamic neuroendocrine neurons in a stepwise fashion, as observed in vivo. These findings indicate that, instead of the addition of inductive signals, minimization of exogenous patterning signaling plays a key role in rostral hypothalamic specification of neural progenitors derived from pluripotent cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
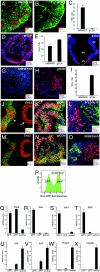
Fig. 1.
SFEBq culture in growth factor-free medium generates rostral hypothalamic progenitors from ES cells. (A, C, E, G, and I) SFEBq-cultured ES cells using KSR-containing medium, (B, C–E, H, I, K, and N) SFEBq-cultured ES cells using gfCDM. Immunostaining analysis of cryostat sections. (F) Coronal section of mouse rostral diencephalon at E10.5. (J and M) Expression in the rostral–dorsal hypothalamic neuroepithelium (arrowhead) and optic cup (arrow) at E10.5. (L and O) SFEB/DLFA-cultured ES cells for retinal differentiation. The sections were stained with antibodies against Bf1 (A and B), Six3 (D), Rax (G, H, J–O), _N_-cadherin (A and B), Nestin (J–L), Sox1 (M–O). DAPI for counter staining. (P–X) SFEBq-cultured Rax–EGFP cells are sorted by FACS (P) and analyzed by qPCR on day 7 for the expression of Otx2 (Q), Rax (R), Vax1 (S), Six3 (T), Irx3 (U), En2 (V), Hoxb1 (W), and Hoxb9 (X). Total RNA from E10.5 whole embryos was used as a control.
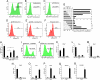
Fig. 2.
Insulin suppresses Rax expression on SFEBq culture. (A–C) FACS analysis of Rax–GFP knocked-in ES cells that were cultured by SFEBq for 7 days in gfCDM (A), GMEM/KSR (B), and gfCDM + 7 μg/ml insulin C. (D–F) Annexin V+ apoptotic cells were analyzed (day 4) with (E) or without (D) insulin. For positive control, cells were preincubated with 10 μM etoposide for 4 h (F). (G) Time-window analysis of insulin's effect on Rax–GFP+ percentages by FACS on day 7. Insulin (7 μg/ml) was removed (upper graph) or added (lower graph) as shown in the left bars. The ratio of the Rax–GFP+ percentages in the presence of insulin to that of SFEBq/gfCDM culture (row 1; referred to as 1.0) is shown in the graphs at the right. (H–J) Effects of inhibitors on SFEBq-cultured ES cells in gfCDM with or without insulin (7 μg/ml) were analyzed by FACS on day 7. LY294002 (H and I), Akt inhibitor VIII (H and J), PD98059 (10 μM), or DMSO (vehicle) was added on day 2. Statistical significance vs. the control (gfCDM + insulin with DMSO) was evaluated by the Dunnette test. *, P < 0.05; **, P < 0.01. (K–R) qPCR analysis of insulin's effect on day 7 was performed as in Fig. 1 P–X.
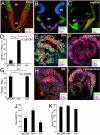
Fig. 3.
ES cell-derived Rax+ progenitors differentiate into early neuronal precursors of the dorsal and ventral hypothalamus. (A–C) Immunohistochemical analysis of the E10.5 mouse brain with Nkx2.1 (A–C), Pax6 (A and B) and Rax (B and C) antibodies. Coronal (A and B) and parasagittal (C). dh, vh, rh, and ch, dorsal, ventral, rostral, and caudal portions of the hypothalamus; ge, ganglionic eminence. The caudal hypothalamus (its ventral portion) is Nkx2.1+ and Rax−. (D–I) Subregional marker expression in SFEBq/gfCDM-cultured ES cells on day 7. Frozen sections of ES cell aggregates treated with 30 nM Shh (D, E, G, and H) or 10 μM cyclopamine (D, F, G, and I) were immunostained for Rax and Nkx2.1 (D and F) or Pax6 (G–I). (D and G) Statistical significance vs. the control (gfCDM) was evaluated by the Dunnette test. *, P < 0.05; **, P < 0.01; ns, not significant. (J and K) Effects of Shh (30 nM) and cyclopamine (10 μM) on the percentages of Rax–GFP+ (J) and Sox1–GFP+ (K) cells in SFEBq/gfCDM culture.
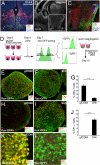
Fig. 4.
ES cell-derived Rx+ progenitors differentiate into early precursor neurons of the dorsal and ventral hypothalamus. (A–C) E13.5 mouse hypothalamus. Coronal (A) and parasagittal (B and C) sections were stained with DAPI and antibodies against Otp (A and C; dorsal domain, d), SF1 (A and C; ventral domain, v), and Foxb1 (C; mb). III, third ventricle; lv, lateral ventricle; or, optic recess; and p, pituitary. (C) Immunostaining within the square (hypothalamic region) in panel B. (D) Schematic of reaggregation culture of FACS-sorted Rax–GFP+ progenitors. (E–J) The aggregates were cultured in DMEM/F12/KSR medium with (F, G, I, J, and L) or without (E, G, H, J, and K) 30 nM Shh (days 7–13). Frozen sections were immunostained for Otp (E–G, K, and L), SF1 (H, I, and J), Tuj (E and F), Nkx2.1 (H and I), and Brn2 (K and L). (G and J) Statistical significance was examined by Mann–Whitney's test. ***, P < 0.001.
Fig. 5.
Generation of characteristic dorsal and ventral hypothalamic neurons. (A) Otp and neurophysin II (NP II) expression in the mouse hypothalamus at E15.5. At this stage, although Otp expression is found predominantly in the dorsal domain, a small number of neurons in ventrally located nuclei such as the lateroanterior hypothalamic nucleus and arcuate nucleus (data not shown) also express Otp. (B–K) Long-term neuronal culture following FACS sorting (day 7) of Rax–GFP+ progenitors from SFEBq/gfCDM-cultured ES cells. (B) Schematic of the culture procedure for rostral hypothalamic differentiation. (C–H) Immunostaining of a Transwell-cultured aggregate cultured without Shh. A number of NP II+ neurons were seen (C and F). High magnification views of NP II+ neurons showed predominantly large somas with a well developed NP II+ Golgi area (D) and characteristic varicosities in their neurites (E). (G) Immunostaining for NP II and synaptophysin in dissociation culture of Rax–GFP+ cells on day 35. (H) AVP-release analysis upon high K+ stimulation on day 25. AVP concentrations in conditioned media of Rax–GFP+ aggregates were measured by RIA. (I–K) Immunostaining of dissociation culture of Rax–GFP+ cells cultured with Shh (day 25). (I) SF1 (green), VGluT2 (located mainly in the growth cones; red), and TuJ1 (white). (J) Immunostaining for TH and Nkx2.1. (K) Immunostaining for AgRP and NPY. (Scale bars, 20 μm.)
Similar articles
- Making pituitary hormone-producing cells in a dish [Review].
Suga H. Suga H. Endocr J. 2016 Aug 31;63(8):669-80. doi: 10.1507/endocrj.EJ16-0232. Epub 2016 May 28. Endocr J. 2016. PMID: 27245938 Review. - Recapitulating Hypothalamus and Pituitary Development Using Embryonic Stem/Induced Pluripotent Stem Cells.
Suga H. Suga H. 2016 Jul 27. In: Pfaff D, Christen Y, editors. Stem Cells in Neuroendocrinology [Internet]. Cham (CH): Springer; 2016. 2016 Jul 27. In: Pfaff D, Christen Y, editors. Stem Cells in Neuroendocrinology [Internet]. Cham (CH): Springer; 2016. PMID: 28590699 Free Books & Documents. Review. - Differentiation of pluripotent stem cells into hypothalamic and pituitary cells.
Suga H. Suga H. Neuroendocrinology. 2015;101(1):18-24. doi: 10.1159/000369821. Epub 2014 Nov 18. Neuroendocrinology. 2015. PMID: 25428763 - Tanycyte-Like Cells Derived From Mouse Embryonic Stem Culture Show Hypothalamic Neural Stem/Progenitor Cell Functions.
Kano M, Suga H, Ishihara T, Sakakibara M, Soen M, Yamada T, Ozaki H, Mitsumoto K, Kasai T, Sugiyama M, Onoue T, Tsunekawa T, Takagi H, Hagiwara D, Ito Y, Iwama S, Goto M, Banno R, Arima H. Kano M, et al. Endocrinology. 2019 Jul 1;160(7):1701-1718. doi: 10.1210/en.2019-00105. Endocrinology. 2019. PMID: 31135891 - Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals.
Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, Sasai Y. Danjo T, et al. J Neurosci. 2011 Feb 2;31(5):1919-33. doi: 10.1523/JNEUROSCI.5128-10.2011. J Neurosci. 2011. PMID: 21289201 Free PMC article.
Cited by
- Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells.
Matsumoto R, Suga H, Aoi T, Bando H, Fukuoka H, Iguchi G, Narumi S, Hasegawa T, Muguruma K, Ogawa W, Takahashi Y. Matsumoto R, et al. J Clin Invest. 2020 Feb 3;130(2):641-654. doi: 10.1172/JCI127378. J Clin Invest. 2020. PMID: 31845906 Free PMC article. - Brain Organoids: Filling the Need for a Human Model of Neurological Disorder.
Jalink P, Caiazzo M. Jalink P, et al. Biology (Basel). 2021 Aug 2;10(8):740. doi: 10.3390/biology10080740. Biology (Basel). 2021. PMID: 34439972 Free PMC article. Review. - Tomosyn Negatively Regulates Arginine Vasopressin Secretion in Embryonic Stem Cell-Derived Neurons.
Takeuchi S, Iwama S, Takagi H, Kiyota A, Nakashima K, Izumida H, Fujisawa H, Iwata N, Suga H, Watanabe T, Kaibuchi K, Oiso Y, Arima H, Sugimura Y. Takeuchi S, et al. PLoS One. 2016 Oct 12;11(10):e0164544. doi: 10.1371/journal.pone.0164544. eCollection 2016. PLoS One. 2016. PMID: 27732637 Free PMC article. - The reprotoxic adverse side effects of neurogenic and neuroprotective drugs: current use of human organoid modeling as a potential alternative to preclinical models.
Abady MM, Jeong JS, Kwon HJ, Assiri AM, Cho J, Saadeldin IM. Abady MM, et al. Front Pharmacol. 2024 Jun 14;15:1412188. doi: 10.3389/fphar.2024.1412188. eCollection 2024. Front Pharmacol. 2024. PMID: 38948466 Free PMC article. Review. - Recapitulating the human segmentation clock with pluripotent stem cells.
Matsuda M, Yamanaka Y, Uemura M, Osawa M, Saito MK, Nagahashi A, Nishio M, Guo L, Ikegawa S, Sakurai S, Kihara S, Maurissen TL, Nakamura M, Matsumoto T, Yoshitomi H, Ikeya M, Kawakami N, Yamamoto T, Woltjen K, Ebisuya M, Toguchida J, Alev C. Matsuda M, et al. Nature. 2020 Apr;580(7801):124-129. doi: 10.1038/s41586-020-2144-9. Epub 2020 Apr 1. Nature. 2020. PMID: 32238941
References
- Tropepe V, et al. Direct neural fate specification from embryonic stem cells: A primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. - PubMed
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. - PubMed
- Watanabe K, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. - PubMed
- Ying QL, et al. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous