Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation - PubMed (original) (raw)
Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation
Juliette Mouriès et al. Blood. 2008.
Abstract
Cross-presentation is a crucial mechanism in tumoral and microbial immunity because it allows internalized cell associated or exogenous antigens (Ags) to be delivered into the major histocompatibility complex I pathway. This pathway is important for the development of CD8(+) T-cell responses and for the induction of tolerance. In mice, cross-presentation is considered to be a unique property of CD8alpha+ conventional dendritic cells (DCs). Here we show that splenic plasmacytoid DCs (pDCs) efficiently capture exogenous Ags in vivo but are not able to cross-present these Ags at steady state. However, in vitro and in vivo stimulation by Toll-like receptor-7, or -9 or viruses licenses pDCs to cross-present soluble or particulate Ags by a transporter associated with antigen processing-dependent mechanism. Induction of cross-presentation confers to pDCs the ability to generate efficient effector CD8+ T-cell responses against exogenous Ags in vivo, showing that pDCs may play a crucial role in induction of adaptive immune responses against pathogens that do not infect tissues of hemopoietic origin. This study provides the first evidence for an in vivo role of splenic pDCs in Ag cross-presentation and T-cell cross-priming and suggests that pDCs may constitute an attractive target to boost the efficacy of vaccines based on cytotoxic T lymphocyte induction.
Figures
Figure 1
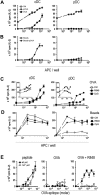
Splenic pDCs capture and degrade Ags efficiently. (A) Spleen cells were incubated for 1 hour in the presence of labeled Ags at 37°C in culture medium (CM) or at 4°C in CM containing 0.1% azide. Cells were stained with anti-CD11c and anti-B220 mAbs and analyzed by flow cytometry. DC subsets were gated as indicated. (B) Top panel: fluorescent intensity of labeled Ags was analyzed for each gated DC subset incubated at 37°C (thick line histogram) or at 4°C in CM containing 0.1% azide (filled histogram). Bottom panel: the percentage of cells gated in M1 that have captured Ag is plotted for each subset. Results represent cumulative data from 4 independent experiments and are expressed as mean percentages plus or minus SD. *P < .05, ***P < .001. (C) Spleen cells were incubated with Lucifer Yellow (0.4 mg/mL) in the presence (dotted line histogram) or absence (thick line histogram) of inhibitor (dimethyl amiloride, 100 μM) at 37°C or at 4°C (filled histogram), and fluorescent intensity was analyzed for both DC subsets. Lucifer Yellow+ cells were gated as shown. (D) Percentage of Lucifer Yellow+ cells in each condition is plotted. Results represent cumulative data from 4 independent experiments and are expressed as mean percentages plus or minus SD.
Figure 2
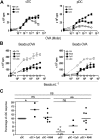
Cross-presentation by pDCs is inducible. (A-D) Various concentrations of purified cDCs (left panels) or pDCs (right panels) were cultured for 24 hours with 105 OVA-specific B3Z cells. (A,B) Cells were incubated in the presence of: (A) culture medium alone (○, CM), OVA (●, 3 mg/mL), OVA257-264 peptide ( pOVA, 1 μg/mL), (B) control beads (◇, 107/mL) or beads-OVA257-264 (♦, 107/mL). (C,D) Cells were incubated in the presence of OVA (3 mg/mL; C, main graph), OVA257-264 peptide (1 μg/mL; C, inset) or beads-OVA257-264 (107/mL, D) and culture medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (E) 104-purified pDCs from TAP−/− or WT mice were incubated in the presence of serial dilution of OVA257-264 peptide (left), OVA alone (middle), or OVA and R848 (right) and 105 B3Z for 24 hours. Stimulation of B3Z was measured as the release of IL-2 into supernatants as assayed by CTLL-2 proliferation monitored by 3H-labeled thymidine incorporation. Results are expressed as mean cpm plus or minus SD for duplicate wells and are representative of 3 experiments.
pOVA, 1 μg/mL), (B) control beads (◇, 107/mL) or beads-OVA257-264 (♦, 107/mL). (C,D) Cells were incubated in the presence of OVA (3 mg/mL; C, main graph), OVA257-264 peptide (1 μg/mL; C, inset) or beads-OVA257-264 (107/mL, D) and culture medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (E) 104-purified pDCs from TAP−/− or WT mice were incubated in the presence of serial dilution of OVA257-264 peptide (left), OVA alone (middle), or OVA and R848 (right) and 105 B3Z for 24 hours. Stimulation of B3Z was measured as the release of IL-2 into supernatants as assayed by CTLL-2 proliferation monitored by 3H-labeled thymidine incorporation. Results are expressed as mean cpm plus or minus SD for duplicate wells and are representative of 3 experiments.
Figure 3
pDCs cross-prime naive T cells. (A) A total of 104 purified splenic cDCs (left panel) or pDCs (right panel) were incubated for 2 hours in the presence of various concentrations of OVA with either medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (B) A total of 104 purified splenic cDCs (gray dotted line) or pDCs (black lines) were incubated for 2 hours in the presence of various concentrations of beads carrying the OVA257-264 peptide (beads-pOVA, left panel) or beads carrying OVA protein (beads-OVA, right panel) with either medium alone (○, CM), R848 (●, 1 μg/mL), or CpG (■, 10 μg/mL). (A,B) Cells were washed twice and cocultured with 2 × 104 OT-I CD8+ transgenic T cells for 72 hours. T-cell proliferation was measured according to 3H-labeled thymidine incorporation during the final 6 hours of culture. Results are expressed as mean cpm plus or minus SD for duplicate wells. One representative experiment of 3 (with CpG) or 7 (with R848) is depicted. (C) For statistical analysis, cumulative data from 3 to 7 independent experiments are plotted. Each dot represents the maximum OT-I response obtained in the presence of a given DC subset and of various doses of OVA. Results are expressed as a percentage of OVA cross-presentation obtained with nonstimulated cDCs (cDC = 100%). ns indicates not significant. *P < .05, **P < .01.
Figure 4
pDCs capture and internalize Ag in vivo. (A,B) Mice were injected intravenously with various numbers of fluorescent red beads. Two hours later, CD11c+ cells enriched from spleen were stained with anti-CD11c and BST-2 (PDCA-1) mAbs and analyzed by flow cytometry. The percentage of cells that had captured beads was determined by gating red-positive cells for pDCs (CD11clow BST-2+ cells, left histogram) and cDCs (CD11chi BST-2− cells, right histogram). One representative experiment of 4 is depicted in each case. (A) Analysis of capture by DCs from the mouse injected with 109 beads is shown. (B) Percentage of bead-positive cells was determined among cDCs (▲,  ) and pDCs (●,
) and pDCs (●,  ) and plotted against the number of red beads injected per mouse. (C) Purified pDCs isolated from (109) red bead-injected mice were stained with anti-BST-2 (120G8, green) mAb and analyzed by fluorescent microscopy (original magnification ×630). Three-dimensional acquisition was done to obtain different slices of the cells. Representative DIC (differential interference contrast), shadow views (projection of each slice in the same image), and 3-dimensional reconstitutions are shown. (D,E) Mice were injected intravenously with various doses of OVA-A488. Two hours later, splenocytes were labeled with anti-CD11c, BST-2 (PDCA-1), and CD3ϵ mAbs and analyzed by flow cytometry. (D) Histograms show OVA-488 fluorescent intensity for both pDCs and cDCs (gated as in panel A) from mice injected with PBS (filled histogram) or 0.2 mg (thin histogram) or 9 mg (thick histogram) OVA. One representative experiment of 3 is depicted. (E) Mean OVA-A488 fluorescence was determined for T cells (○, black line), cDCs (▲,
) and plotted against the number of red beads injected per mouse. (C) Purified pDCs isolated from (109) red bead-injected mice were stained with anti-BST-2 (120G8, green) mAb and analyzed by fluorescent microscopy (original magnification ×630). Three-dimensional acquisition was done to obtain different slices of the cells. Representative DIC (differential interference contrast), shadow views (projection of each slice in the same image), and 3-dimensional reconstitutions are shown. (D,E) Mice were injected intravenously with various doses of OVA-A488. Two hours later, splenocytes were labeled with anti-CD11c, BST-2 (PDCA-1), and CD3ϵ mAbs and analyzed by flow cytometry. (D) Histograms show OVA-488 fluorescent intensity for both pDCs and cDCs (gated as in panel A) from mice injected with PBS (filled histogram) or 0.2 mg (thin histogram) or 9 mg (thick histogram) OVA. One representative experiment of 3 is depicted. (E) Mean OVA-A488 fluorescence was determined for T cells (○, black line), cDCs (▲,  ), and pDCs (●,
), and pDCs (●,  ) and is plotted against the dose of OVA-488 injected. T cells were gated as CD3ϵ+ cells. (F) Purified pDCs isolated from mice injected with PBS or 3 or 9 mg OVA-A488 (green) were stained with anti-BST-2 (120G8, red) and CD11c (yellow) mAbs and Hoechst 44 432 (light blue) and then analyzed by fluorescent microscopy (original magnification ×630). One representative experiment of 3 is depicted.
) and is plotted against the dose of OVA-488 injected. T cells were gated as CD3ϵ+ cells. (F) Purified pDCs isolated from mice injected with PBS or 3 or 9 mg OVA-A488 (green) were stained with anti-BST-2 (120G8, red) and CD11c (yellow) mAbs and Hoechst 44 432 (light blue) and then analyzed by fluorescent microscopy (original magnification ×630). One representative experiment of 3 is depicted.
Figure 5
OVA captured in vivo is cross-presented by pDCs after in vitro stimulation. Mice were injected intravenously with OVA (9 mg). Two hours later, pDCs and cDCs were purified and various concentrations of both DC subsets were cocultured with either culture medium alone (CM), R848 (1 μg/mL), or CpG (10 μg/mL) and 2.5 × 104 CFSE-labeled OT-I T cells. CFSE profiles of OT-I T cells were analyzed by flow cytometry 72 hours later. (A) CFSE profiles obtained with 2 × 105 DCs are shown for both DC subsets and for each conditions as indicated. Proliferating OT-I T cells are gated in M1. (B) Percentages of gated proliferating T cells are plotted according to the number of pDCs (top panel) or cDCs (bottom panel) per well in culture medium (○, CM), or in the presence of R848 (●) or CpG (■). One representative experiment of 3 is depicted.
Figure 6
pDCs cross-prime OT-I T cells in vivo. 129sv mice were injected intravenously with OVA (9 mg) and either PBS, 10 μg of R848, 100 μg CpG in DOTAP, 103 hemagglutinin units of heat-inactivated Influenza A virus (IAV), or 106 pfu of BV. A control group received only PBS. Two hours later, splenic DC subsets were purified by magnetic sorting and their capacity to cross-prime OT-I T cells in vitro (A,C) and in vivo (B,D) was assessed. (A,C) Various numbers of purified cDCs (A, left panel) and pDCs (A, right panel; C) were cultured with 2.5 × 104 CFSE-labeled OT-I T cells. OT-I T-cell proliferation was analyzed by flow cytometry 72 hours later. Proliferating T cells were assessed by CFSE dye dilution by OT-I T cells as described in Figure 5. Percentages of proliferating T cells are plotted against the number of DCs per well. (A) cDCs (left panel) and pDCs (right panel) were purified from mice injected with either OVA alone (○) or with R848 (●) or with CpG (■). (C) pDCs were purified from mice injected with either OVA alone (○) or with BV (●) or with IAV (■). Left panel: 1 representative experiment of 3 is depicted. Right panel: statistical analysis of OT-I T-cell proliferation, when cocultured with 2 × 105 purified pDCs, from 3 independent experiments, including 1 or 2 mice per group in each experiment. (B,D) Purified pDCs were injected into CD45.1 hosts that had received 2 × 106 CFSE-labeled OT-I T (CD45.2) cells 24 hours before. Seven days later, splenocytes from these mice were incubated 4 hours in the presence of brefeldin A with or without OVA257-264 peptide. Cells were stained using anti-CD45.1, CD45.2, CD3ϵ, and CD8α mAbs, and OT-I T cells were characterized as CD45.1−, CD45.2+, CD8α+, and CD3ϵ+ cell populations. (B) OT-I T-cell analysis from mice that received pDCs purified from mice injected with PBS or with OVA and PBS, CpG, or R848 as indicated. CD44 expression and intracellular expression of IFN-γ by OT-I T cells were assessed as described in “Adoptive transfer.” First row: CFSE profiles of OT-I T cells (percentages of OT-I T cells showing CFSE dye dilution and gated in M1 are indicated); second row: CD44 expression on OT-I T cells according to CFSE profile; third row: IFN-γ intracellular staining on OT-I T cells according to CFSE profile; last row: intracellular staining with control isotype. (D) CFSE profiles of OT-I T cells from mice that received pDCs purified from mice injected with PBS or with OVA and PBS, BV, or IAV. First row: percentages of OT-I T cells showing CFSE dye dilution and gated in M1. Second row: CD44 expression by OT-I cells according to CFSE profiles. Third row: IFN-γ intracellular staining on OT-I cells according to CFSE profile; last row: intracellular staining with control isotype. One representative experiment of 4 is depicted.
Figure 7
Cross-priming of a primary CD8+ T response by R848-activated pDCs. Naive 129sv mice were immunized by 2 consecutive intravenous injections (at days 0 and 7) of pDCs or cDCs purified from mice injected with either PBS alone or OVA (9 mg) and 10 μg R848. Spleen from immunized mice were analyzed 1 week after the last immunization. (A) Splenocytes were labeled with anti-CD90, anti-CD8α mAbs, and H2-Kb-SIINFEKL-pentamer and analyzed by flow cytometry. First row: CD8α+ CD90+cells were gated among total T cells. Second row: Kb-SIINFEKL-pentamer+ cells were gated among CD8+ T cells. Percentages of gated cells are indicated in each quadrant. (B,C) Splenocytes were restimulated with OVA peptide (1 μg/mL) for 24 hours and with brefeldin A for the last 4 hours. Cells were then labeled with anti-CD5, anti-CD8α mAbs, and H2-Kb-SIINFEKL-pentamer. Intracellular anti–IFN-γ staining was performed before analysis by flow cytometry. CD8α+ cells were gated among total T cells and percentage of IFN-γ+ within CD5+ T cells is shown. IFN-γ is plotted against H2-Kb-SIINFEKL-pentamer to visualize costaining (B). Pooled results showing percentages of IFN-γ+ cells among CD8+ T cells from 2 independent experiments are plotted (C). (D) Specific lysis of OVA257-264–loaded cells was assayed by in vivo killing. Results from individual mice are shown for each group.
Similar articles
- TLR7 triggering with polyuridylic acid promotes cross-presentation in CD8α+ conventional dendritic cells by enhancing antigen preservation and MHC class I antigen permanence on the dendritic cell surface.
Crespo MI, Zacca ER, Núñez NG, Ranocchia RP, Maccioni M, Maletto BA, Pistoresi-Palencia MC, Morón G. Crespo MI, et al. J Immunol. 2013 Feb 1;190(3):948-60. doi: 10.4049/jimmunol.1102725. Epub 2013 Jan 2. J Immunol. 2013. PMID: 23284054 - Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes.
Fu C, Peng P, Loschko J, Feng L, Pham P, Cui W, Lee KP, Krug AB, Jiang A. Fu C, et al. Proc Natl Acad Sci U S A. 2020 Sep 22;117(38):23730-23741. doi: 10.1073/pnas.2002345117. Epub 2020 Sep 2. Proc Natl Acad Sci U S A. 2020. PMID: 32879009 Free PMC article. - Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets.
Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, Lambeck AJ, Figdor CG, de Vries IJ. Tel J, et al. Blood. 2013 Jan 17;121(3):459-67. doi: 10.1182/blood-2012-06-435644. Epub 2012 Dec 4. Blood. 2013. PMID: 23212525 - Induction of antigen cross-presentation by Toll-like receptors.
Datta SK, Raz E. Datta SK, et al. Springer Semin Immunopathol. 2005 Jan;26(3):247-55. doi: 10.1007/s00281-004-0174-2. Epub 2004 Oct 14. Springer Semin Immunopathol. 2005. PMID: 15609002 Review. - Unique functions of splenic CD8alpha+ dendritic cells during infection with intracellular pathogens.
Neuenhahn M, Busch DH. Neuenhahn M, et al. Immunol Lett. 2007 Dec 15;114(2):66-72. doi: 10.1016/j.imlet.2007.09.007. Epub 2007 Oct 12. Immunol Lett. 2007. PMID: 17964665 Review.
Cited by
- Gut redox and microbiome: charting the roadmap to T-cell regulation.
Prasad S, Singh S, Menge S, Mohapatra I, Kim S, Helland L, Singh G, Singh A. Prasad S, et al. Front Immunol. 2024 Aug 21;15:1387903. doi: 10.3389/fimmu.2024.1387903. eCollection 2024. Front Immunol. 2024. PMID: 39234241 Free PMC article. Review. - Dendritic Cell-Hitchhiking In Vivo for Vaccine Delivery to Lymph Nodes.
Zhou L, Zhao L, Wang M, Qi X, Zhang X, Song Q, Xue D, Mao M, Zhang Z, Shi J, Si P, Liu J. Zhou L, et al. Adv Sci (Weinh). 2024 Sep;11(33):e2402199. doi: 10.1002/advs.202402199. Epub 2024 Jul 4. Adv Sci (Weinh). 2024. PMID: 38962939 Free PMC article. - A Cancer Nanovaccine for Co-Delivery of Peptide Neoantigens and Optimized Combinations of STING and TLR4 Agonists.
Baljon JJ, Kwiatkowski AJ, Pagendarm HM, Stone PT, Kumar A, Bharti V, Schulman JA, Becker KW, Roth EW, Christov PP, Joyce S, Wilson JT. Baljon JJ, et al. ACS Nano. 2024 Mar 5;18(9):6845-6862. doi: 10.1021/acsnano.3c04471. Epub 2024 Feb 22. ACS Nano. 2024. PMID: 38386282 Free PMC article. - The Autoinducer N-Octanoyl-L-Homoserine Lactone (C8-HSL) as a Potential Adjuvant in Vaccine Formulations.
Shah SM, Joshi D, Chbib C, Roni MA, Uddin MN. Shah SM, et al. Pharmaceuticals (Basel). 2023 May 8;16(5):713. doi: 10.3390/ph16050713. Pharmaceuticals (Basel). 2023. PMID: 37242496 Free PMC article. - The stimulatory effect of fusobacteria on dendritic cells under aerobic or anaerobic conditions.
Koido S, Horiuchi S, Kan S, Bito T, Ito Z, Uchiyama K, Saruta M, Sato N, Ohkusa T. Koido S, et al. Sci Rep. 2022 Jun 23;12(1):10698. doi: 10.1038/s41598-022-14934-z. Sci Rep. 2022. PMID: 35739324 Free PMC article.
References
- Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–288. - PubMed
- Boisgerault F, Rueda P, Sun CM, Hervas-Stubbs S, Rojas M, Leclerc C. Cross-priming of T-cell responses by synthetic microspheres carrying a CD8+ T cell epitope requires an adjuvant signal. J Immunol. 2005;174:3432–3439. - PubMed
- Li M, Davey GM, Sutherland RM, et al. Cell-associated ovalbumin is cross-presented much more efficiently than soluble ovalbumin in vivo. J Immunol. 2001;166:6099–6103. - PubMed
- Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials