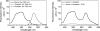Use of multidimensional fluorescence resonance energy transfer to establish the orientation of cholecystokinin docked at the type A cholecystokinin receptor - PubMed (original) (raw)
. 2008 Sep 9;47(36):9574-81.
doi: 10.1021/bi800734w. Epub 2008 Aug 13.
Affiliations
- PMID: 18700727
- PMCID: PMC3648886
- DOI: 10.1021/bi800734w
Use of multidimensional fluorescence resonance energy transfer to establish the orientation of cholecystokinin docked at the type A cholecystokinin receptor
Kaleeckal G Harikumar et al. Biochemistry. 2008.
Abstract
Fluorescence resonance energy transfer (FRET) represents a powerful tool to establish relative distances between donor and acceptor fluorophores. By utilizing several donors situated in distinct positions within a docked full agonist ligand and several acceptors distributed at distinct sites within its receptor, multiple interdependent dimensions can be determined. These can provide a unique method to establish or confirm three-dimensional structure of the molecular complex. In this work, we have utilized full agonist analogues of cholecystokinin (CCK) with Aladan distributed throughout the pharmacophore in positions 24, 29, and 33, along with receptor constructs derivatized with Alexa (546) at positions 94, 102, 204, and 341 in the helical bundle and first, second, and third extracellular loops, respectively. These provided 12 FRET distances to overlay on working models of the CCK-occupied receptor. These established that the carboxyl terminus of CCK resides at the external surface of the lipid bilayer, adjacent to the receptor amino-terminal tail, rather than being inserted into the helical bundle. They also provide important experimentally derived constraints for understanding spatial relationships between the docked ligand and the flexible extracellular loop regions. Multidimensional FRET provides a new independent method to establish and refine structural insights into ligand-receptor complexes.
Figures
Figure 1
Control spectra. Shown are representative emission spectra of a fluorescence donor (Aladan24-CCK) bound to a monoreactive CCK receptor construct (N102C) after excitation with 362 nm light, and that of the same receptor derivatized with the fluorescence acceptor, Alexa546, after excitation with light at either 362 nm or at 546 nm (left panel). This clearly establishes that the wavelength of light used to excite the donor has no ability to excite the acceptor in the absence of energy transfer from donor to acceptor. Also shown is the elimination of the FRET signal when nonfluorescent CCK (1 _μ_M) competed for occupation of this monoreactive receptor by the fluorescence donor (right panel). This establishes the critical nature of spatial approximation between donor and acceptor provided by receptor occupation of donor in the experimental paradigm.
Figure 2
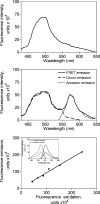
Experimental spectra. Shown are representative data generated with cells stably expressing the null-cysteine-reactive CCK receptor construct (top panel) and a representative pseudowild type, monocysteine-reactive CCK receptor construct (N102C) (middle panel). The cells were derivatized with the Alexa546-MTS reagent and subsequently allowed to bind fluorescent Aladan ligand, as described in Methods. Cells were then excited by light at 362 nm and the illustrated emission spectra were collected. A peak at 572 nm in the monoreactive receptor-bearing cells indicates significant fluorescence resonance energy transfer, while the null-cysteine-reactive CCK receptor-bearing cells did not have such a peak. Also shown in the middle panel is the ability to deconvolute the FRET emission spectrum into its component donor emission and acceptor emission spectra. The bottom panel shows the calibration of Alexa fluorescence acceptor excitation and acceptor emission spectra, permitting the determination of the acceptor excitation associated with any observed acceptor emission. The Pearson correlation coefficient, “_r_”, for the relationship between values of acceptor excitation and acceptor emission was 0.99.
Figure 3
Molecular model of the CCK-occupied CCK receptor. Shown are views from the side (left panel) and top (right panel) of the molecular model of the CCK-occupied CCK receptor that is based on photoaffinity labeling data (14). This model is fully compatible with all twelve of the current experimentally derived FRET distance constraints. Highlighted in dashed white lines are the four distances between the C_β_ positions of the fluorescence donor at the critical residue at the carboxyl terminus of CCK (Phe33) and the sites of the fluorescence acceptors within the CCK receptor. These distances best distinguish the two divergent working models of the CCK-occupied receptor, with the carboxyl terminus of the peptide situated in substantially different places in the two models. The backbone of the CCK ligand is illustrated in blue-to-red from amino terminus to carboxyl terminus. The sites of fluorescence donors are expanded and labeled. Each of the extracellular loop (ECL) regions of the CCK receptor is labeled. The disulfide bond that links extracellular loops one and two is illustrated as well.
Similar articles
- Measurement of intermolecular distances for the natural agonist Peptide docked at the cholecystokinin receptor expressed in situ using fluorescence resonance energy transfer.
Harikumar KG, Pinon DI, Wessels WS, Dawson ES, Lybrand TP, Prendergast FG, Miller LJ. Harikumar KG, et al. Mol Pharmacol. 2004 Jan;65(1):28-35. doi: 10.1124/mol.65.1.28. Mol Pharmacol. 2004. PMID: 14722234 - Fluorescence resonance energy transfer analysis of the antagonist- and partial agonist-occupied states of the cholecystokinin receptor.
Harikumar KG, Miller LJ. Harikumar KG, et al. J Biol Chem. 2005 May 13;280(19):18631-5. doi: 10.1074/jbc.M410834200. Epub 2005 Mar 9. J Biol Chem. 2005. PMID: 15757907 - Therapeutic potential for novel drugs targeting the type 1 cholecystokinin receptor.
Cawston EE, Miller LJ. Cawston EE, et al. Br J Pharmacol. 2010 Mar;159(5):1009-21. doi: 10.1111/j.1476-5381.2009.00489.x. Epub 2009 Nov 18. Br J Pharmacol. 2010. PMID: 19922535 Free PMC article. Review. - Structure of cholecystokinin receptor binding sites and mechanism of activation/inactivation by agonists/antagonists.
Fourmy D, Escrieut C, Archer E, Galès C, Gigoux V, Maigret B, Moroder L, Silvente-Poirot S, Martinez J, Fehrentz JA, Pradayrol L. Fourmy D, et al. Pharmacol Toxicol. 2002 Dec;91(6):313-20. doi: 10.1034/j.1600-0773.2002.910608.x. Pharmacol Toxicol. 2002. PMID: 12688374 Review.
Cited by
- Fluorescent approaches for understanding interactions of ligands with G protein coupled receptors.
Sridharan R, Zuber J, Connelly SM, Mathew E, Dumont ME. Sridharan R, et al. Biochim Biophys Acta. 2014 Jan;1838(1 Pt A):15-33. doi: 10.1016/j.bbamem.2013.09.005. Epub 2013 Sep 18. Biochim Biophys Acta. 2014. PMID: 24055822 Free PMC article. Review. - Molecular Mechanism of Action of Triazolobenzodiazepinone Agonists of the Type 1 Cholecystokinin Receptor. Possible Cooperativity across the Receptor Homodimeric Complex.
Desai AJ, Lam PC, Orry A, Abagyan R, Christopoulos A, Sexton PM, Miller LJ. Desai AJ, et al. J Med Chem. 2015 Dec 24;58(24):9562-77. doi: 10.1021/acs.jmedchem.5b01110. Epub 2015 Dec 10. J Med Chem. 2015. PMID: 26654202 Free PMC article. - Regulation of acinar cell function in the pancreas.
Williams JA. Williams JA. Curr Opin Gastroenterol. 2010 Sep;26(5):478-83. doi: 10.1097/MOG.0b013e32833d11c6. Curr Opin Gastroenterol. 2010. PMID: 20625287 Free PMC article. Review. - Cholecystokinin-induced satiety, a key gut servomechanism that is affected by the membrane microenvironment of this receptor.
Desai AJ, Dong M, Harikumar KG, Miller LJ. Desai AJ, et al. Int J Obes Suppl. 2016 Dec;6(Suppl 1):S22-S27. doi: 10.1038/ijosup.2016.5. Epub 2016 Nov 16. Int J Obes Suppl. 2016. PMID: 28685026 Free PMC article. Review. - Elucidation of the molecular basis of cholecystokinin Peptide docking to its receptor using site-specific intrinsic photoaffinity labeling and molecular modeling.
Dong M, Lam PC, Pinon DI, Abagyan R, Miller LJ. Dong M, et al. Biochemistry. 2009 Jun 16;48(23):5303-12. doi: 10.1021/bi9004705. Biochemistry. 2009. PMID: 19441839 Free PMC article.
References
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. - PubMed
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. - PubMed
- Ji TH, Grossmann M, Ji I. G protein-coupled receptors. I. Diversity of receptor-ligand interactions. J. Biol. Chem. 1998;273:17299–17302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources