TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment - PubMed (original) (raw)
TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment
Yun Li et al. Neuron. 2008.
Erratum in
- Neuron. 2008 Nov 26;60(4):730
Abstract
Adult hippocampal neurogenesis is stimulated by chronic administration of antidepressants (ADs) and by voluntary exercise. Neural progenitor cells (NPCs) in the dentate gyrus (DG) that are capable of continuous proliferation and neuronal differentiation are the source of such structural plasticity. Here we report that mice lacking the receptor tyrosine kinase TrkB in hippocampal NPCs have impaired proliferation and neurogenesis. When exposed to chronic ADs or wheel-running, no increase in proliferation or neurogenesis is observed. Ablation of TrkB also renders these mice behaviorally insensitive to antidepressive treatment in depression- and anxiety-like paradigms. In contrast, mice lacking TrkB only in differentiated DG neurons display typical neurogenesis and respond normally to chronic ADs. Thus, our data establish an essential cell-autonomous role for TrkB in regulating hippocampal neurogenesis and behavioral sensitivity to antidepressive treatments, and support the notion that impairment of the neurogenic niche is an etiological factor for refractory responses to an antidepressive regimen.
Figures
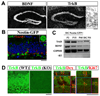
Figure 1. TrkB is expressed by hippocampal NPCs
(A) In situ hybridization analysis of TrkB and BDNF mRNA in the adult dentate gyrus (DG). In the high magnification image (right), note the distribution of silver gram (black spheres) in all cells. Scale bars, 1mm (low-magnification) and 10um (high-magnification). GL, granular layer; SGZ, sub-granular zone. (B) Confocal image of the DG of an adult Nestin-GFP transgenic mouse, co-immunostained for GFP (green), NeuroD (red) and NeuN (blue). GFP expression was restricted to NPCs and did not colocalize with immature (NeuroD+) or mature (NeuN+) neurons. Insert showed a DG derived neurosphere that expresses GFP. Scale bars, 10um. (C) RT-PCR detection of TrkB and BDNF transcripts in FACS sorted Nestin-GFP positive cells and DG derived neurospheres. NS, neurosphere. (D) Immuno-staining for TrkB (green) on adult DG sections from wild-type and TrkBhGFAP mice (left panels). Co-staining for TrkB (green) and Ki67 (red), or Doublecortin (red) demonstrated co-localization of TrkB with proliferating (Ki67+) and differentiating (Doublecortin+) cells. Dcx, doublecortin. Scale bars, 10um and 5um. WT, wild-type.
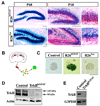
Figure 2. hGFAP-Cre, but not Syn-Cre, mediated recombination in hippocampal NPCs
(A) X-gal staining on DG sections of R26RhGFAP and R26Syn mice at P10 and P60. Higher magnification views of the circled areas are shown in the right panels, in which black lines outline the granular layer, while the red arrow highlights the SGZ and inner granular layer where recombination was spared. Scale bars, 100um. (B) Schematic diagram of the procedure to generate neurospheres from adult DG. (C) X-gal staining on primary neurospheres derived from the DG of adult control, R26RhGFAP and R26Syn mice. Blue staining in the neurosphere from R26RhGFAP mice indicated the occurrence of recombination. Scale bar, 100um. (D) Western blots of lysates from the hippocampus of control and TrkBhGFAP mice, probed for TrkB and actin. Note the absence of TrkB in the TrkBhGFAP mice. (E) RT-PCR detection of TrkB and G3PDH transcripts in FACS sorted Nestin-GFP positive cells from the DG of Control and TrkBhGFAP mice. Note the absence of TrkB in the TrkBhGFAP mice.
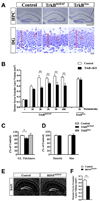
Figure 3. Ablation of TrkB in postnatal NPCs impaired DG morphogenesis
(A) Representative images of Nissl stained DG sections from control, TrkBhGFAP and TrkBSyn mice at P15. The decreases in DG size and granular layer thickness were only observed in TrkBhGFAP mice. Red lines and circles highlight the length of the granular layer, and the size of single cell, respectively. Scale bars, 1mm (hippocampus) and 10um (DG). (B) Quantitative analysis revealed that the reduction in the DG volume of TrkBhGFAP mice first became measurable at P10 days and persisted in adulthood. Results are mean + SEM here and in subsequent figures; n>7 for each. (C) At P15 days, TrkBhGFAP, but not TrkBSyn mice had thinner granular layer, demonstrated a decrease in neuronal number. Data was shown as percentage of control. N=6 for each. F2,15=5.477, p=0.0164. GL, granular layer. (D) Comparative analysis on the size and density of DG granular neurons from control and TrkBhGFAP mice. Note the absence of difference in either category. (E) DAPI staining of hippocampus sections from control and BDNFhGFAP mice at the age of 2 month. Note the decrease in DG size in the BDNFhGFAP mice. Scale bar, 200um. (F) Quantitative analysis revealed a 30% reduction in DG volume in the BDNFhGFAP mice at the age of 2 months. N=8–10 for each. * p<0.05; ** p<0.01
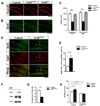
Figure 4. Lack of TrkB in NPCs impaired neurogenesis and proliferation in vivo and in vitro
(A) Representative confocal images of the DG immunostained for NeuroD (red). Note the reduction of NeuroD positive cells, representing immature neurons, in TrkBhGFAP, but not TrkBSyn mice (P15). Scale bar, 100um. (B–C) Proliferation in the DG was decreased in TrkBhGFAP, but not TrkBSyn mice, evidence by a reduction in Ki67 positive (C), or BrdU positive cells (B and C). Scale bar, 100um. N=7–9 for each. F2,21=78.39, p<0.0001 (BrdU); F2,21=51.64, p<0.0001 (Ki67). (D–E) Cell cycle analysis using BrdU pulsing and co-immunostaining for BrdU (green) and Ki67 (red) showed increase in the ratio of BrdU labeled cells that have exited the cell cycle (BrdU+Ki67−) in the DG of TrkBhGFAP mice. Arrowheads indicate BrdU+Ki67− cells. Scale bar, 100um. Data represents the ratio of (BrdU+Ki67−) /(All BrdU+). (F) Western blots of lysates from DG derived neurospheres probed for phosphor-Trk490, TrkB and actin, with and without BDNF stimulation. Note the abundance of TrkB expression and the increase of phospho-Trk in the presence of BDNF. (G) Cells from the DG of adult mice were plated at equal density and allowed to proliferate in the presence of EGF and bFGF. The frequency of neurosphere formation was lower in the TrkBhGFAP mice, indicating a decrease in NPC population. (H) Addition of BDNF facilitated the growth of primary neurospheres derived from the DG of control mice, but not the TrkBhGFAP mice. TrkBhGFAP neurospheres grown without BDNF were also smaller than control, indicated impaired proliferation. N=4 for each. ANOVA revealed significant effects of BDNF (F1,12=6.994, p=0.0214), genotype (F1,12=102.2, p<0.0001) and an interaction between the two (F1,12=19.3, p=0.0009). *p<0.05; **p<0.01; ***p<0.001.
Figure 5. TrkB expression in NPCs was required for chronic ADs induced proliferation and neurogenesis
(A) Representative confocal images of immunostaining for Ki67 in the DG of saline or fluoxetine treated mice. Chronic treatment with fluoxetine increased the number of proliferating cells (Ki67+) in the DG of control, TrkBSyn, but not TrkBhGFAP mice. Scale bar, 100um. Sal, saline; Flx, fluoxetine. (B) Quantitative analysis of Ki67 positive cells demonstrated lack of increases in the TrkBhGFAP mice after fluoxetine or imipramine treatment. Note the lower number of Ki67 positive cells in the TrkBhGFAP mice with either saline or AD, indicating impairment in proliferation at both basal and stimulated level. N=7–25 for each. ANOVA (GLM) found significant effects of AD treatment (F2.98=40.48, p<0.0001), genotype (F2,98=332.3, p<0.0001) and the interaction of both (F2,98=19.64, p<0.0001). Imi, imipramine. (C) Chronic fluoxetine treatment increased the number of Doublecortin positive cells (red) in the DG of control and TrkBSyn but not TrkBhGFAP mice. Scale bar, 10um. (D) Fluoxetine and imipramine failed to elicit an increase in the number of immature neurons (Doublecortin+) in the TrkBhGFAP mice. N=7–25 for each. ANOVA (GLM) revealed significant effects of AD treatment (F2,98=30.79, p<0.0001), genotype (F2,98=211.6, p<0.0001) and interaction between the two (F2,98=5.471, p=0.0005). ***p<0.001.
Figure 6. TrkBhGFAP mice were insensitive to chronic AD and exercise induced improvement in depression and anxiety-like behaviors
(A) In the novelty-suppressed feeding test (NSFT), chronic treatments with fluoxetine or imipramine shortened the latency to feed (indicating reduced anxiety) in control, TrkBSyn but not TrkBhGFAP mice. Data were shown as percentage of control with saline injection. N=6–29 for each group. ANOVA (GLM) found significant effects of AD treatment (F2,100=8.022, p=0.0006) and genotype (F2,100=10.49, p<0.0001). (B) The tail-suspension test (TST) measured total duration of immobility (“behavior despair”), which could be reduced by chronic fluoxetine or imipramine in control, TrkBSyn but not TrkBhGFAP mice. Data were shown as percentage of control with saline injection. N=7–28 for each. ANOVA (GLM) revealed significant effects of AD treatment (F2,100=4.233, p=0.0172), genotype (F2,100=20.03, p<0.0001) and the interaction of both (F2,100=5.085, p=0.0009). (C–D) Running for 6 weeks failed to increase the number of Ki67 (C) or Doublecortin (D) positive cells in the DG of TrkBhGFAP mice. N=6–13 for each. ANOVA (GLM) revealed significant effects of running (F1,36=13.64, p=0.0007 for Ki67; F1,36=16.01, p=0.0003 for Doublecortin), genotype (F1,36=141.1, p<0.0001 for Ki67; F1,36=58.46, p<0.0001 for Doublecortin) and the interaction of the two (F1,36=12.66, p=0.0010 for Ki67; F1,36=14.79, p=0.0005 for Doublecortin). (E–F) TrkBhGFAP mice did not display decrease in latency to feed (E, NSFT) or duration of immobility (F, TST) after 6 weeks of running, compared to sedentary controls. Data were shown as percentage of sedentary control. N=8–16 for each. NSFT (F1,41=15.09, p=0.0004 for genotype), TST (F1,41=9.082, p=0.0044 for genotype; F1,41=8.273, p=0.0064 for exercise). *p<0.05; **p<0.01; ***p<0.001.
Figure 7. Specific ablation of TrkB from adult NPCs was sufficient to block AD sensitivity
(A) X-gal staining on sagittal brain sections of R26Nestin mice, treated with vehicle or tamoxifen at 1 month and analyzed at 2 months of age. (B) X-gal staining on DG sections of R26Nestin mice, treated with tamoxifen at 1 month and analyzed 1 month or 6 months afterwards. Note the increase in X-gal stained cells 6 months after tamoxifen injection. Scale bar, 200um. (C) Co-staining for β-gal (green) and Doublecortin (red) on DG sections from R26Nestin mice, treated with tamoxifen at 1 month and analyzed at 4 months of age. Scale bar, 10um. (D) RT-PCR detection of TrkB and G3PDH transcripts in FACS sorted Nestin-GFP positive and negative cells from the DG of TrkBflox/flox; Nestin-CreER mice treated with tamoxifen (TrkBNestin) or vehicle (control) at 1 month old. (E) Co-staining for TrkB (green) and Ki67 (red) on DG sections from TrkBNestin mice at the age of 3 months (2 months post-tamoxifen injection), note the lack of co-localization of TrkB and Ki67 in the SGZ. Scale bars, 10um and 5um. (F) TrkBNestin mice at the age of 3 months had normal DG volume. (G–H) Quantitative analysis of Ki67 (G) and Doublecortin (H) positive cells demonstrated lack of increases in the TrkBNestin mice after fluoxetine or imipramine treatments. N=8–10 for each. ANOVA (GLM) found significant effects of AD treatment (F2,50=12.45, p<0.0001 for Ki67; F2,50=7.014, p=0.0021 for Doublecortin), genotype (F1,50=176.7, p<0.0001 for Ki67; F1,50=141.9, p<0.0001 for Doublecortin) and an interaction of the two (F2,50=13.57, p<0.0001 for Ki67; F2,50=12.56, p<0.0001 for Doublecortin). (I–J) TrkBNestin mice did not display decrease in latency to feed (I, NSFT) or duration of immobility (J, TST) after chronic exposure to fluoxetine or imipramine, compared to control mice. Data were shown as percentage of control treated with saline. N=8–10 for each. NSFT: F1,50=13.68, p=0.0005 for genotype, F2,50=4.206, p=0.0205 for the interaction of genotype and AD treatment. TST: F1,50=18.15, p<0.0001 for genotype, F2,50=6.848, p=0.0024 for AD and F2,50=9.488, p=0.0003 for an interaction of the two. *p<0.05. ***p<0.001.
Comment in
- Keeping 'trk' of antidepressant actions.
Banasr M, Duman RS. Banasr M, et al. Neuron. 2008 Aug 14;59(3):349-51. doi: 10.1016/j.neuron.2008.07.028. Neuron. 2008. PMID: 18701059
Similar articles
- Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus.
Sairanen M, Lucas G, Ernfors P, Castrén M, Castrén E. Sairanen M, et al. J Neurosci. 2005 Feb 2;25(5):1089-94. doi: 10.1523/JNEUROSCI.3741-04.2005. J Neurosci. 2005. PMID: 15689544 Free PMC article. - Chronic Lithium Treatment Enhances the Number of Quiescent Neural Progenitors but Not the Number of DCX-Positive Immature Neurons.
Kara N, Narayanan S, Belmaker RH, Einat H, Vaidya VA, Agam G. Kara N, et al. Int J Neuropsychopharmacol. 2015 Jan 29;18(7):pyv003. doi: 10.1093/ijnp/pyv003. Int J Neuropsychopharmacol. 2015. PMID: 25636892 Free PMC article. - The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain.
Kim HJ, Leeds P, Chuang DM. Kim HJ, et al. J Neurochem. 2009 Aug;110(4):1226-40. doi: 10.1111/j.1471-4159.2009.06212.x. Epub 2009 Jun 15. J Neurochem. 2009. PMID: 19549282 Free PMC article. - Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise.
Micheli L, Ceccarelli M, D'Andrea G, Tirone F. Micheli L, et al. Brain Res Bull. 2018 Oct;143:181-193. doi: 10.1016/j.brainresbull.2018.09.002. Epub 2018 Sep 17. Brain Res Bull. 2018. PMID: 30236533 Review. - Antidepressants and Cdk inhibitors: releasing the brake on neurogenesis?
Chesnokova V, Pechnick RN. Chesnokova V, et al. Cell Cycle. 2008 Aug;7(15):2321-6. doi: 10.4161/cc.6446. Epub 2008 Jun 17. Cell Cycle. 2008. PMID: 18682686 Free PMC article. Review.
Cited by
- The intricate interplay between microglia and adult neurogenesis in Alzheimer's disease.
Früholz I, Meyer-Luehmann M. Früholz I, et al. Front Cell Neurosci. 2024 Sep 18;18:1456253. doi: 10.3389/fncel.2024.1456253. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39360265 Free PMC article. Review. - Stimulating myelin restoration with BDNF: a promising therapeutic approach for Alzheimer's disease.
Zota I, Chanoumidou K, Gravanis A, Charalampopoulos I. Zota I, et al. Front Cell Neurosci. 2024 Sep 2;18:1422130. doi: 10.3389/fncel.2024.1422130. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39285941 Free PMC article. Review. - Adult Neurogenesis, Learning and Memory.
Šimončičová E, Henderson Pekarik K, Vecchiarelli HA, Lauro C, Maggi L, Tremblay MÈ. Šimončičová E, et al. Adv Neurobiol. 2024;37:221-242. doi: 10.1007/978-3-031-55529-9_13. Adv Neurobiol. 2024. PMID: 39207695 Review. - The Neurotrophin System in the Postnatal Brain-An Introduction.
von Bohlen Und Halbach O, Klausch M. von Bohlen Und Halbach O, et al. Biology (Basel). 2024 Jul 24;13(8):558. doi: 10.3390/biology13080558. Biology (Basel). 2024. PMID: 39194496 Free PMC article. Review. - The intersection between menopause and depression: overview of research using animal models.
Herrera-Pérez JJ, Hernández-Hernández OT, Flores-Ramos M, Cueto-Escobedo J, Rodríguez-Landa JF, Martínez-Mota L. Herrera-Pérez JJ, et al. Front Psychiatry. 2024 Jul 15;15:1408878. doi: 10.3389/fpsyt.2024.1408878. eCollection 2024. Front Psychiatry. 2024. PMID: 39081530 Free PMC article. Review.
References
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. - PubMed
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. - PubMed
- Alvarez-Buylla A. Neurogenesis and plasticity in the CNS of adult birds. Exp Neurol. 1992;115:110–114. - PubMed
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH066172/MH/NIMH NIH HHS/United States
- R37 NS033199-08/NS/NINDS NIH HHS/United States
- R37 NS033199-10/NS/NINDS NIH HHS/United States
- R37 NS033199/NS/NINDS NIH HHS/United States
- R37NS033199/NS/NINDS NIH HHS/United States
- P50 MH066172-070002/MH/NIMH NIH HHS/United States
- P50MH66172/MH/NIMH NIH HHS/United States
- R37 NS033199-11/NS/NINDS NIH HHS/United States
- K08 HD001470/HD/NICHD NIH HHS/United States
- R37 NS033199-09/NS/NINDS NIH HHS/United States
- P50 MH066172-060002/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases