Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes - PubMed (original) (raw)
Beta2-integrin-induced p38 MAPK activation is a key mediator in the CD14/TLR4/MD2-dependent uptake of lipopolysaccharide by hepatocytes
Melanie J Scott et al. J Biol Chem. 2008.
Abstract
The liver is the main organ that clears circulating lipopolysaccharide (LPS), and hepatocytes are a major cell type involved in LPS uptake. Little is known about the mechanisms for LPS internalization in hepatocytes and what signaling pathways are involved. We show here that LPS uptake is initiated after formation of a multi-receptor complex within lipid rafts. We find that essential components for LPS uptake are CD14, TLR4, MD2, and the beta2-integrin CD11b/CD18. Activation of p38 MAPK is also essential for the initiation of LPS uptake, and interestingly, we show that this activation is not through TLR4 signaling by MyD88 but through activation of TIRAP via CD11b/CD18. However, TLR4/MD2 remain essential components at the cell surface as part of the LPS receptor complex. We therefore suggest novel roles for TLR4/MD2, CD11b/CD18, TIRAP, and p38 MAPK in LPS uptake by hepatocytes.
Figures
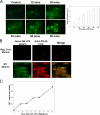
FIGURE 1.
Hepatocytes take up LPS. A, uptake of fluorescent LPS up to 90 min in primary isolated mouse hepatocytes from C57BL/6 (WT) mice. The cells were fixed and visualized by Olympus Provis fluorescent microscopy (×40 magnification) (left panels). Fluorescence was determined relative to base-line background using Metamorph™ software (n = 4/time point) (right panel). B, comparison of LPS uptake in isolated hepatocytes using Alexa Fluor™ E. coli LPS (green) with additional immunostaining with anti-E. coli antibody (red). Negative control (Neg. Cont.) received no fluorescent LPS but was immunostained as above. The merged images show good correlation of green and red fluorescence in the images. C, images representative of three separate experiments. WT (C57BL/6) hepatocytes were given 100 ng/ml radioactive labeled 14C-LPS for time points up to 90 min. The cell lysates were then analyzed for radioactivity by scintillation counting. The error bars show S.E.
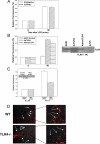
FIGURE 2.
Uptake of LPS in isolated hepatocytes is CD14- and TLR4-dependent. A, fluorescent LPS uptake in hepatocytes from WT, CD14-/-, or TLR4-/- mice. Fluorescence is relative to background determined by Olympus Provis fluorescent microscopy (×40 magnification) (n = 4/time point, representative images). B, left panel, LPS uptake after 90 min in CD14-/- hepatocytes pretreated for 24 h with control virus (AdΨ5) or virus expressing full-length CD14 (AdCD14) or TLR4-/- hepatocytes pretreated for 24 h with control virus or virus expressing TLR4 (AdTLR4). Right panels, expression of CD14 and TLR4 confirmed by immunoblot. CD14-/- hepatocytes were pretreated with 1 μg of recombinant soluble CD14 (sCD14) for 1 h before the addition of fluorescent LPS for 90 min. C, uptake of LPS was visualized by fluorescent microscopy, and relative quantitation was performed using Metamorph™. D, hepatocytes were isolated from WT (C57BL/10) and TLR4-/- hepatocytes and treated with 100 ng/ml S. minnesota LPS for 90 min. Hepatocytes were isolated from WT (C57BL/10) and TLR4-/- hepatocytes. Cell culture medium was replaced with media containing 10% fetal calf serum. E, fluorescent LPS (100 ng/ml) was then added for 90 min, and LPS uptake was visualized by fluorescent microscopy. Relative quantitation of LPS uptake was done using Metamorph™.
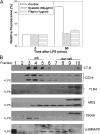
FIGURE 3.
LPS uptake in hepatocytes is not dependent on TLR4 signaling through TIR domain. A, uptake of fluorescent LPS in hepatocytes from C3H/HeOuJ and C3H/HeJ (TLR4 mutant) mice. B, left panel, LPS uptake at 90 min in hepatocytes from TLR4-/- mice pretreated for 48 h with adenovirus expressing TLR4 (AdTLR4), mutant TLR4 (AdmutTLR4), or control (AdΨ5) (×40 magnification, n = 4/experimental group per time point). Right panel, expression of TLR4 confirmed by immunoblot. C, LPS uptake at 90 min in WT hepatocytes pretreated with siRNA targeted against MD2 or a nontargeting control. Inset, knockdown of MD2 confirmed by immunoblot of whole cell lysates. D, isolated hepatocytes from WT and TLR4-/- mice immunostained for MD2 (Cy3, red). The images were taken using confocal microscopy in phase contrast with merged MD2 staining (Cy3, red). The white arrows indicate positions of cell membrane as shown by the phase contrast image. The right panels are magnified portions of the left panels.
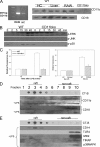
FIGURE 4.
LPS uptake in hepatocytes is dependent on TIRAP and p38 MAPK activation. A, LPS uptake at 90 min in WT hepatocytes pretreated for 24 h with either siRNA targeting MyD88 or control nontargeting siRNA or pretreated for 24 h with MyD88 inhibitory peptide or control peptide for 24 h.Lower panel, knockdown of MyD88 with siRNA confirmed by immunoblot.B, LPS uptake in WT hepatocytes pretreated with either TIRAP siRNA or control nontargeting siRNA for 24 h or pretreated with TIRAP inhibitory peptide or control peptide for 24 h. Lower panel, knockdown of TIRAP with siRNA confirmed by immunoblot. C, LPS uptake in WT (C57BL/6) and MyD88-/- hepatocytes. D, left panel, Western blot of whole cell lysates from isolated WT hepatocytes pretreated for 24 h with either MyD88, TIRAP, or control siRNA ± LPS for 15 min and immunoblotted for phospho-ERK, total ERK phospho-p38, or total p38 MAPK.Right panel, relative band density for Western blot: pERK and pp38 compared with control. E, Western blot of whole cell lysates from WT, CD14-/-, TLR4-/-, C3H/HeOuJ, and C3H/HeJ hepatocytes to identify activation (phosphorylation) of p38 MAPK and ERK after 100 ng/ml_E. coli_ LPS up to 45 min. F, left panel, inhibition of p38 MAPK by pretreatment with the inhibitor SB203580, but not treatment with JNK inhibitor or U0126 (MEK1/2 inhibitor), prevented uptake of Alexa Fluor™ E. coli LPS (100 ng/ml) into WT hepatocytes (n = 4/experimental group per time point). Right panels, LPS uptake in hepatocytes from WT mice pretreated for 48 h with adenovirus expressing dominant negative p38 MAPK (AdDNp38) or control (AdΨ5) (×40 magnification, images representative of four separate sets of experiments). Expression of p38 was confirmed by immunoblot.
FIGURE 5.
LPS uptake in hepatocytes is lipid raft-dependent. A, LPS uptake at 90 min in WT hepatocytes isolated from WT mice pretreated with lipid raft disruptors nystatin, filipin, or control (Me2SO) for 1 h (n = 4/group/time point). B, lipid raft fractions isolated by ultracentrifugation in a discontinuous sucrose gradient from hepatocyte cell lysates, ± 100 ng/ml E. coli LPS for 15 min. Fractions containing lipid rafts were identified by Western dot-blot using horseradish peroxidase-cholera toxin subunit B (CT-B). Western blot of WT hepatocyte fractions separated by SDS-PAGE was immunoblotted for CD14, TLR4, MD2, TIRAP, p38 MAPK ± LPS for 15 min.
FIGURE 6.
Activation of p38 MAPK requires β2-integrins and β2-integrin localization to lipid rafts. A, left panel, CD11b mRNA expression in RAW cells (positive control) and mouse WT hepatocytes (HC) using reverse transcription-PCR. Right panel, CD18 and CD11b protein expression by Western blot in RAW cells (positive control), WT liver tissue lysates, and cell lysates from isolated WT and CD11b-/- hepatocytes. B, Western blot of whole cell lysates from WT and CD11b-/- (CD11bko) hepatocytes to show activation (phosphorylation) of ERK, JNK, and p38 MAPK after addition of 100 ng/ml E. coli LPS. LPS uptake at 90 min in hepatocytes isolated from WT, CD11b-/-, and CD18-deficient mice. C, the cells were fixed and visualized by fluorescent microscopy, and fluorescence relative to background was determined (n = 4/group/time point). D, lipid raft fractions isolated from WT hepatocytes ± LPS for 15 min separated by SDS-PAGE and immunoblotted for CD11b or CD18. E, lipid raft fractions from CD11b-/- hepatocytes ± LPS for 15 min separated by SDS-PAGE and immunoblotted for CD14, TLR4, MD2, TIRAP, and p38 MAPK.
Similar articles
- Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism.
Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Scott MJ, et al. Hepatology. 2009 May;49(5):1695-708. doi: 10.1002/hep.22839. Hepatology. 2009. PMID: 19296467 Free PMC article. - Lipopolysaccharide (LPS) directly suppresses growth hormone receptor (GHR) expression through MyD88-dependent and -independent Toll-like receptor-4/MD2 complex signaling pathways.
Dejkhamron P, Thimmarayappa J, Kotlyarevska K, Sun J, Lu C, Bonkowski EL, Denson LA, Menon RK. Dejkhamron P, et al. Mol Cell Endocrinol. 2007 Aug 15;274(1-2):35-42. doi: 10.1016/j.mce.2007.05.013. Epub 2007 May 29. Mol Cell Endocrinol. 2007. PMID: 17601656 Free PMC article. - Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition.
Erridge C, Kennedy S, Spickett CM, Webb DJ. Erridge C, et al. J Biol Chem. 2008 Sep 5;283(36):24748-59. doi: 10.1074/jbc.M800352200. Epub 2008 Jun 17. J Biol Chem. 2008. PMID: 18559343 Free PMC article. - LPS induction of gene expression in human monocytes.
Guha M, Mackman N. Guha M, et al. Cell Signal. 2001 Feb;13(2):85-94. doi: 10.1016/s0898-6568(00)00149-2. Cell Signal. 2001. PMID: 11257452 Review. - Toll-like receptor and tumour necrosis factor dependent endotoxin-induced acute lung injury.
Togbe D, Schnyder-Candrian S, Schnyder B, Doz E, Noulin N, Janot L, Secher T, Gasse P, Lima C, Coelho FR, Vasseur V, Erard F, Ryffel B, Couillin I, Moser R. Togbe D, et al. Int J Exp Pathol. 2007 Dec;88(6):387-91. doi: 10.1111/j.1365-2613.2007.00566.x. Int J Exp Pathol. 2007. PMID: 18039275 Free PMC article. Review.
Cited by
- Current understanding of metformin effect on the control of hyperglycemia in diabetes.
An H, He L. An H, et al. J Endocrinol. 2016 Mar;228(3):R97-106. doi: 10.1530/JOE-15-0447. Epub 2016 Jan 7. J Endocrinol. 2016. PMID: 26743209 Free PMC article. Review. - The Battle of LPS Clearance in Host Defense vs. Inflammatory Signaling.
Kumar P, Schroder EA, Rajaram MVS, Harris EN, Ganesan LP. Kumar P, et al. Cells. 2024 Sep 21;13(18):1590. doi: 10.3390/cells13181590. Cells. 2024. PMID: 39329771 Free PMC article. Review. - Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism.
Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Scott MJ, et al. Hepatology. 2009 May;49(5):1695-708. doi: 10.1002/hep.22839. Hepatology. 2009. PMID: 19296467 Free PMC article. - Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis.
Guo J, Friedman SL. Guo J, et al. Fibrogenesis Tissue Repair. 2010 Oct 21;3:21. doi: 10.1186/1755-1536-3-21. Fibrogenesis Tissue Repair. 2010. PMID: 20964825 Free PMC article. - The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis.
Deng M, Tang Y, Li W, Wang X, Zhang R, Zhang X, Zhao X, Liu J, Tang C, Liu Z, Huang Y, Peng H, Xiao L, Tang D, Scott MJ, Wang Q, Liu J, Xiao X, Watkins S, Li J, Yang H, Wang H, Chen F, Tracey KJ, Billiar TR, Lu B. Deng M, et al. Immunity. 2018 Oct 16;49(4):740-753.e7. doi: 10.1016/j.immuni.2018.08.016. Epub 2018 Oct 9. Immunity. 2018. PMID: 30314759 Free PMC article.
References
- Mathison, J. C., and Ulevitch, R. J. (1979) J. Immunol. 123 2133-2143 - PubMed
- Hopf, U., Ramadori, G., Moller, B., and Galanos, C. (1984) Am. J. Emerg. Med. 2 13-19 - PubMed
- Mimura, Y., Sakisaka, S., Harada, M., Sata, M., and Tanikawa, K. (1995) Gastroenterology 109 1969-1976 - PubMed
- Kmiec, Z. (2001) Adv. Anat. Embryol. Cell Biol. 161 1-151 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials