Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice - PubMed (original) (raw)
Comparative Study
Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice
Suzanne E Hickman et al. J Neurosci. 2008.
Abstract
Early microglial accumulation in Alzheimer's disease (AD) delays disease progression by promoting clearance of beta-amyloid (Abeta) before formation of senile plaques. However, persistent Abeta accumulation despite increasing microglial numbers suggests that the ability of microglia to clear Abeta may decrease with age and progression of AD pathology. To determine the effects of aging and Abeta deposition on microglial ability to clear Abeta, we used quantitative PCR to analyze gene expression in freshly isolated adult microglia from 1.5-, 3-, 8-, and 14-month-old transgenic PS1-APP mice, an established mouse model of AD, and from their nontransgenic littermates. We found that microglia from old PS1-APP mice, but not from younger mice, have a twofold to fivefold decrease in expression of the Abeta-binding scavenger receptors scavenger receptor A (SRA), CD36, and RAGE (receptor for advanced-glycosylation endproducts), and the Abeta-degrading enzymes insulysin, neprilysin, and MMP9, compared with their littermate controls. In contrast, PS1-APP microglia had a 2.5-fold increase in the proinflammatory cytokines IL-1beta (interleukin-1beta) and tumor necrosis factor alpha (TNFalpha), suggesting that there is an inverse correlation between cytokine production and Abeta clearance. In support of this possibility, we found that incubation of cultured N9 mouse microglia with TNFalpha decreased the expression of SRA and CD36 and reduced Abeta uptake. Our data indicate that, although early microglial recruitment promotes Abeta clearance and is neuroprotective in AD, as disease progresses, proinflammatory cytokines produced in response to Abeta deposition downregulate genes involved in Abeta clearance and promote Abeta accumulation, therefore contributing to neurodegeneration. Antiinflammatory therapy for AD should take this dichotomous microglial role into consideration.
Figures
Figure 1.
Increased number of senile-like plaques and plaque-associated microglia with aging in transgenic PS1-APP mice. Top panels, Frozen sections from transgenic PS1-APP brains stained with pan-α-Aβ antibody (which recognizes all forms of β-amyloid) show (original magnification, 40×) sparse amounts of Aβ in a transgenic mouse at 3 months of age (A) and large numbers of Aβ deposits in a 14-month-old transgenic mouse distributed throughout cortex and hippocampus (B). The inset in B shows the plaque signified by an arrow magnified to 400× original magnification. Bottom panels, 400× original magnification. Microglia are stained using α-CD11b antibody (reddish brown Nova red development) and Aβ-containing plaques are stained with thioflavin-S (green). In a 3-month-old mouse, plaques are rarely seen and microglia are evenly distributed throughout the hippocampus region shown in C. D, In contrast, florid plaques are seen in a transgenic mouse at 14 months of age, and intensely stained microglia are clustered around the plaques. The staining was repeated in six mice in each age group with similar staining.
Figure 2.
Gene expression profile of adult WT CD11b+ microglia and CD11bneg cells (endothelial cells and astrocytes) measured by QPCR. A, Protocol used for isolating CD11b+ cells from adult mouse brains. B, CD11b+ cells (black bars) have high levels of CD11b RNA and barely detectable levels of cell markers for astrocytes (GFAP) and endothelial cells (CD31) confirming high purity of the CD11b+ cells preparations. The CD11bneg population (gray bars) expresses CD31 and GFAP, but no CD11. C, CD11b+ cells isolated using our protocol expressed SRA, SRB1, and CD36 (3 known microglia receptors), some RAGE, and negligible levels of MARCO.
Figure 3.
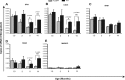
Reduced expression of Aβ-binding receptors in microglia from old transgenic PS1-APP mice. Expression of Aβ-binding receptors in CD11b+ cells was compared between transgenic PS1-APP mice and their age-matched WT littermates at 1.5, 3, 8, and 14 months of age. Data represent the average values obtained by QPCR on six sets of mice for 3 and 14 months of age and on five sets of mice at 1.5 and 8 months of age. *Values of p are shown only if differences are significant (<0.05). A, B, D, At 8 and 14 months of age, PS1-APP transgenic mice show significantly reduced expression of SRA (A), CD36 (B), and RAGE (D) compared with their WT littermates. C, There are no significant differences in expression of SRB1 observed between transgenic and WT mice at any age. E, There was negligible expression of MARCO. Error bars indicate SEM.
Figure 4.
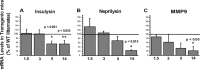
Decreased expression of Aβ-degrading enzymes in microglia from old transgenic PS1-APP mice. Expression of Aβ-degrading enzymes in CD11b+ cells from transgenic PS1-APP mice was compared with their age-matched wild-type littermates at 1.5, 3, 8, and 14 months of age. Data are expressed for transgenic mice as percentage expression of WT. *Values of p are only shown if differences are significant. A–C, Expression of insulysin (A), neprilysin (B), and MMP9 (C), enzymes thought to degrade Aβ, is comparable across WT and transgenic microglia from mice at 1.5 and 3 months of age. A, In 8-month-old PS1-APP transgenic mice, there is a statistically significant reduction only in insulysin RNA levels. However, by 14 months of age, the reduction in expression of all three enzymes is statistically significant (A–C). The horizontal lines across the graphs indicate 100% WT levels. Error bars indicate SEM.
Figure 5.
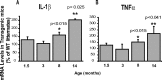
Increased expression of IL-1β and TNFα in microglia from old transgenic PS1-APP mice. A, B, Expression of IL-1β (A) and TNFα (B) was measured by QPCR in CD11b+ cells from transgenic PS1-APP mice and their age-matched, nontransgenic littermates at 1.5, 3, 8, and 14 months of age. Data are displayed for transgenic mice as percentage expression of their WT littermates at the same ages. In young mice (1.5 and 3 months of age), expression of IL-1β (A) and TNFα (B) is comparable across all groups, but at 8 and 14 months of age there are significant increases (p < 0.05) in RNA expression of both IL-1β and TNFα in transgenic compared with WT mice. Error bars indicate SEM.
Figure 6.
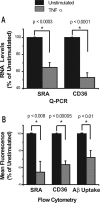
Treatment of N9 cells with TNFα decreases expression of SRA and CD36 and reduces uptake of Aβ. A, B, N9 microglia-like cells were treated with TNFα overnight, and expression of SRA and CD36 were measured by QPCR (A) and by cell surface staining and flow cytometry (B). Data represent the average of five separate experiments and are displayed for treated cells as percentage expression (%) of untreated controls (100%). B, Far right, Results of TNF-α treatment on binding/uptake of Aβ-labeled with Hylite fluor-488. TNF-α-treated or untreated N9 cells were incubated with fluorescent Aβ for 2 h, and then cell-associated fluorescence was measured by flow cytometry. Data represent an average of five experiments and are displayed for TNFα-treated cells as percentage (%) of mean fluorescence intensity of untreated cells. Error bars indicate SEM.
Similar articles
- Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.
Sebastian Monasor L, Müller SA, Colombo AV, Tanrioever G, König J, Roth S, Liesz A, Berghofer A, Piechotta A, Prestel M, Saito T, Saido TC, Herms J, Willem M, Haass C, Lichtenthaler SF, Tahirovic S. Sebastian Monasor L, et al. Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article. - Heterozygous CX3CR1 Deficiency in Microglia Restores Neuronal β-Amyloid Clearance Pathways and Slows Progression of Alzheimer's Like-Disease in PS1-APP Mice.
Hickman SE, Allison EK, Coleman U, Kingery-Gallagher ND, El Khoury J. Hickman SE, et al. Front Immunol. 2019 Dec 2;10:2780. doi: 10.3389/fimmu.2019.02780. eCollection 2019. Front Immunol. 2019. PMID: 31849963 Free PMC article. - Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer's mouse model: Implication of TLR4 signaling in disease progression.
Go M, Kou J, Lim JE, Yang J, Fukuchi KI. Go M, et al. Biochem Biophys Res Commun. 2016 Oct 14;479(2):331-337. doi: 10.1016/j.bbrc.2016.09.073. Epub 2016 Sep 15. Biochem Biophys Res Commun. 2016. PMID: 27641666 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Microglial Aβ receptors in Alzheimer's disease.
Yu Y, Ye RD. Yu Y, et al. Cell Mol Neurobiol. 2015 Jan;35(1):71-83. doi: 10.1007/s10571-014-0101-6. Epub 2014 Aug 23. Cell Mol Neurobiol. 2015. PMID: 25149075 Review.
Cited by
- Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration.
Ernest James Phillips T, Maguire E. Ernest James Phillips T, et al. Front Cell Neurosci. 2021 Mar 26;15:652593. doi: 10.3389/fncel.2021.652593. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33841102 Free PMC article. Review. - PPARγ/RXRα-induced and CD36-mediated microglial amyloid-β phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice.
Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT. Yamanaka M, et al. J Neurosci. 2012 Nov 28;32(48):17321-31. doi: 10.1523/JNEUROSCI.1569-12.2012. J Neurosci. 2012. PMID: 23197723 Free PMC article. - Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: rat and human studies identify strikingly similar changes.
Sárvári M, Hrabovszky E, Kalló I, Solymosi N, Likó I, Berchtold N, Cotman C, Liposits Z. Sárvári M, et al. J Neuroinflammation. 2012 Dec 3;9:264. doi: 10.1186/1742-2094-9-264. J Neuroinflammation. 2012. PMID: 23206327 Free PMC article. - The immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in Alzheimer's disease.
Ye Y, Gao M, Shi W, Gao Y, Li Y, Yang W, Zheng X, Lu X. Ye Y, et al. Front Immunol. 2024 Jan 8;14:1325530. doi: 10.3389/fimmu.2023.1325530. eCollection 2023. Front Immunol. 2024. PMID: 38259476 Free PMC article. Review. - Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-β 1-42 induced neurotoxicity and memory impairment.
Bellozi PM, Lima IV, Dória JG, Vieira ÉL, Campos AC, Candelario-Jalil E, Reis HJ, Teixeira AL, Ribeiro FM, de Oliveira AC. Bellozi PM, et al. Sci Rep. 2016 May 4;6:25226. doi: 10.1038/srep25226. Sci Rep. 2016. PMID: 27142962 Free PMC article.
References
- Alarcón R, Fuenzalida C, Santibáñez M, von Bernhardi R. Expression of scavenger receptors in glial cells. Comparing the adhesion of astrocytes and microglia from neonatal rats to surface-bound beta-amyloid. J Biol Chem. 2005;280:30406–30415. - PubMed
- Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–945. - PubMed
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous