G protein-coupled receptor-promoted trafficking of Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism mediated by Gbeta1gamma2-Rab11a interaction - PubMed (original) (raw)
G protein-coupled receptor-promoted trafficking of Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism mediated by Gbeta1gamma2-Rab11a interaction
Alejandro García-Regalado et al. Mol Biol Cell. 2008 Oct.
Abstract
G-protein coupled receptors activate heterotrimeric G proteins at the plasma membrane in which most of their effectors are intrinsically located or transiently associated as the external signal is being transduced. This paradigm has been extended to the intracellular compartments by studies in yeast showing that trafficking of Galpha activates phosphatidylinositol 3-kinase (PI3K) at endosomal compartments, suggesting that vesicle trafficking regulates potential actions of Galpha and possibly Gbetagamma at the level of endosomes. Here, we show that Gbetagamma interacts with Rab11a and that the two proteins colocalize at early and recycling endosomes in response to activation of lysophosphatidic acid (LPA) receptors. This agonist-dependent association of Gbetagamma to Rab11a-positive endosomes contributes to the recruitment of PI3K and phosphorylation of AKT at this intracellular compartment. These events are sensitive to the expression of a dominant-negative Rab11a mutant or treatment with wortmannin, suggesting that Rab11a-dependent Gbetagamma trafficking promotes the activation of the PI3K/AKT signaling pathway associated with endosomal compartments. In addition, RNA interference-mediated Rab11a depletion, or expression of a dominant-negative Rab11a mutant attenuated LPA-dependent cell survival and proliferation, suggesting that endosomal activation of the PI3K/AKT signaling pathway in response to Gbetagamma trafficking, via its interaction with Rab11, is a relevant step in the mechanism controlling these fundamental events.
Figures
Figure 1.
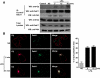
Gβ1γ2 interacts with Rab11a and they colocalize in HEK-293T cells. (A) A full-length clone of Rab11a was identified as a Gβ1 subunit interactor by yeast two-hybrid. The specificity of the interaction between Rab11a and Gβ1 was determined in the yeast two-hybrid system using pGB3 or p53 as negative controls. Yeast grew on media lacking leucine and tryptophan (−LT, which selects for the presence of plasmids; left), but only those displaying interactions grew in restrictive media lacking adenine, histidine, leucine, and tryptophan and were positive for the activity of α-galactosidase (−AHLT + X α-Gal; right). (B) Pull-down analysis in mammalian cells demonstrating the interaction between Gβγ and Rab11a. HEK-293T cells were transfected with GFP-Rab11a (expression detected in total lysates with GFP-specific antibodies; bottom) and His6-tagged Gβ1γ2, 2 d after transfection, His6-Gβ1γ2 was isolated from cells grown in DMEM supplemented with 10% fetal bovine serum by using Talon beads (top), and Rab11a was detected with GFP-specific antibodies. The presence of His-tagged Gβ1 and His-tagged Gγ2 in pull-downs (middle) was detected using an antibody recognizing the poly-histidine tag. NT, nontransfected cells. (C) Interaction between endogenous Gβγ and Rab11 in serum-stimulated HEK-293T. Rab11 was immunoprecipitated from cells grown to 80% of confluence in DMEM supplemented with 10% fetal bovine serum, the presence of Gβ in Rab11 immunoprecipitates and total cell lysate was detected by Western blot analysis (top and third panels, respectively). The presence of Rab11 in the immunoprecipitate and total lysate is shown in the second and fourth panels, respectively. Control refers to immunoprecipitation performed with a rabbit preimmune serum instead of the anti-Rab11 antibody. (D) Confocal images showing the distribution of His6-Gβ1γ2 and GFP-Rab11a in HEK-293 cells. Cells were transiently transfected with His6-Gβ1γ2 either in the absence or presence of GFP-Rab11a, fixed, and stained with anti-histidine antibody and analyzed by confocal microscopy. i, His6-Gβ1γ2 in the absence and in the presence (ii–iv) of GFP-Rab11a. ii, His6-Gβ1γ2 in the presence of GFP-Rab11a. iii, the same image showing GFP-Rab11a. iv, merged images showing colocalization of His6-Gβ1γ2 and GFP-Rab11a. Bar, 8 μm (i).
Figure 2.
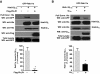
Agonist-dependent interaction between endogenous Gβγ and Rab11 and effect on their subcellular localization. Rab11 was immunoprecipitated from serum-starved HEK-293T cells grown to 80% confluence and stimulated with 10 μM LPA or left without stimulus (NS) as indicated. (A) The presence of Gβ, Rab11, and AKT in Rab11 immunoprecipitates and total lysates was detected by Western blot analysis (IP:Rab11 and total lysates, respectively). Control refers to immunoprecipitation performed with anti-Grb2 antibody instead of the anti-Rab11 antibody. (B) Left, confocal images showing the distribution of Gβ and Rab11a upon LPA stimulation. HEK-293T cells were serum starved and stimulated with 10 μM LPA for the indicated times or left unstimulated (NS), fixed and stained with anti-Gβ antibody and anti-Rab11 antibody as described in Materials and Methods and analyzed by confocal microscopy. Confocal images show the distribution of Gβ in NS cells or at selected times after stimulation with 10 μM LPA. Gβ distribution in NS cells is consistent with its presence preferentially on the cell surface and in some Rab11-positive vesicles. Stimulation with LPA for 5 or 15 min promoted Gβ internalization, which was detected in Rab11-positive endosomes. Bars, 8 and 10 μm, respectively. Right, percentage of colocalization area was determined using the public domain NIH ImageJ (
http://rsb.info.nih.gov/nih-image/
). NS cells, gray bar. LPA-stimulated cells, black bars.
Figure 3.
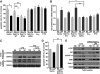
Gβ1γ2 shows a higher affinity for a constitutively active Rab11a Q70L mutant but does not affect the GTPase activity of recombinant Rab11a. HEK-293T cells were transfected with His6-Gβ1γ2 and the indicated amounts of GFP-tagged constructs of wild-type, constitutively active (Q70L), or dominant-negative (S25N) Rab11a; His6-Gβ1γ2 was isolated by pull-down from cells grown in DMEM supplemented with 10% fetal bovine serum by using Talon resin. (A) The GFP-fusion constructs of Rab11a were detected by an anti-GFP antibody in the pull-down (top, left) or in the total lysate (bottom, left), and the presence of His6-Gβ1γ2 in the pull-down was determined by an anti-His antibody. Right graph shows the densitometric values of GFP-Rab11a and its mutants as detected in Gβγ pull-downs, averaged from three independent experiments (means ± SEM). (B) Rab11a GTPase assay. His6-Rab11a was expressed and purified from E. coli BL21. His6-Gβ1γ2 was expressed and isolated from HEK-293T as described in Materials and Methods. Both proteins were incubated to interact overnight followed by incubation with GTP for 30 min. GTPase activity was assessed by a colorimetric method; the samples were read at 620 nm. Insets show isolated His6-Gβ1γ2 (left) and His6-Rab11a (induced or not with 0.1 μM of IPTG; right) used in the experiment. (C) Confocal images showing the distribution of His6-Gβ1γ2 and GFP-Rab11a mutants in HEK-293T cells transiently transfected with His6-Gβ1γ2 and the indicated GFP-Rab11 constructs. i–iii, show the distribution of His6-Gβ1γ2 (i), Rab11a Q70L (ii), and the merged images (iii), whereas (iv–vi) show the equivalent experiments in the presence of Rab11a S25N. Bars, 8 μm.
Figure 4.
Expression of Gαi2 or PhLP1 prevent the interaction between Gβ1γ2 and Rab11a. HEK-293T were transfected with His6-Gβ1γ2 and GFP-Rab11a either in the presence or absence of Flag-tagged PhLP1 or Gαi2. The interaction between His6-Gβ1γ2 and GFP-Rab11a was determined by pull-down assays using Talon resin. (A) Expression of Flag-tagged PhLP1 inhibits the interaction between Gβ1γ2 and GFP-Rab11a (top). Bottom graph represents the mean densitometric values of GFP-Rab11a bound to Gβγ obtained in three independent experiments (means ± SEM, *p < 0.05 compared with the absence of PhPL1 assessed by t test analysis). (B) Expression of Gαi2 significantly reduces the interaction between His6-Gβ1γ2 and GFP-Rab11a (top). Bottom graph shows the results of densitometric analysis of GFP-Rab11a bound to Gβγ obtained in three independent experiments (means ± SEM, *p < 0.05 compared with the absence of PhPL1 assessed by t test analysis). The presence in the pull-downs of His6-Gβ1γ2 and the competing proteins (Gαi2 or PhLP1) and the presence of transfected GFP-Rab11a in total lysates was demonstrated by Western blot.
Figure 5.
LPA stimulation increases Gβ1γ2 association to Rab11a-positive endosomes. HEK-293T cells grown to 80% confluence were serum starved for 15 h followed by stimulation with 10 μM LPA for the indicated times. Cells were fractionated to isolate early and late endosomes as described in Materials and Methods. (A and D) LPA stimulation induces a time-dependent association of Gβ1γ2 heterodimer with Rab11-positive endosomal fractions as detected by Western blotting using an anti-Gβ1 antibody. Distribution of Rab11, Rab5, early endosomal marker EEA1, and late endosomal marker Rab7 was assessed by Western blot as indicated. NS, nonstimulated cells; TL, total cell lysates. The effect of LPA stimulation on the association of PI3-kinase-γ and the presence and endosomal phosphorylation of AKT was also evaluated in the same endosomal fractions by using specific antibodies. (B) Graph represents the mean densitometric values of Gβ1 present in the endosomal fractions determined in three independent experiments. Means ± SEM, *p < 0.001 compared with nonstimulated cells (analysis of variance and Student-Newman-Keuls test). (C) Results of similar densitometric analysis of AKT phosphorylation (means ± SEM, *p < 0.001 compared with nonstimulated cells. (E) Wortmannin prevented LPA-induced endosomal recruitment of PI3-kinase-γ and phosphorylation of AKT. HEK-293T cells were incubated with 300 nM wortmannin for 1 h before LPA stimulation, and early endosomes were isolated as described, and proteins were detected by Western blot using anti-phospho-Ser473-AKT (top), total AKT (middle), and PI3-kinase-γ catalytic subunit (bottom). (F) Wortmannin does not affect LPA-dependent ERK phosphorylation. Total cell lysates were obtained from HEK-293T cells pretreated with wortmannin before LPA stimulation, and the presence of the indicated proteins was detected by Western blotting using specific antibodies.
Figure 6.
Expression of dominant-negative Rab11a, Gαi2, PhLP1, or PTX treatment attenuated the effect of LPA on endosomal recruitment of Gβγ, PI3-kinase-γ, and phosphorylation of AKT. (A) HEK-293T cells transiently transfected with dominant-negative GFP-Rab11a mutant (GFP-Rab11a S25N) were serum starved for 15 h followed by stimulation with 10 μM LPA for the indicated times. Cell lysates were obtained and early endosomal fraction was isolated by a flotation step gradient as described in Materials and Methods. The presence of the indicated proteins in the endosomal fraction was detected by Western blotting using specific antibodies. (B) Effect of different Rab11a constructs on LPA-dependent Gβγ and downstream effectors association to endosomes. HEK-293T cells were transiently transfected with GFP-Rab11a, GFP-Rab11a Q70L, or GFP-Rab11a S25N mutants, cells were processed as described, and the presence of the indicated proteins in the endosomal fraction was detected by Western blotting using specific antibodies. NS, nonstimulated cells. The effect of LPA stimulation on the association of PI3-kinase-γ and the presence and endosomal phosphorylation of AKT was also evaluated in the same fractions by using specific antibodies. (C) Effect of PTX on Gβγ and downstream effector association to Rab11-positive endosomes. HEK-293T cells grown to 80% confluence were serum starved for 15 h followed by stimulation with 10 μM LPA for the indicated times or left unstimulated (NS). A group of cells were incubated with 100 ng/ml PTX for 20 h. The presence of the indicated proteins in the endosomal fraction was detected by Western blotting using specific antibodies. NS, nonstimulated cells. The effect of LPA stimulation and PTX treatment on association of PI3-kinase-γ and the presence and endosomal phosphorylation of AKT were also evaluated in the same endosomal fractions by using specific antibodies. To assess the phosphorylation of AKT in samples with similar amounts of endosomal AKT, we diluted the samples obtained from LPA-stimulated cells (1:4 or 1:1.8, as indicated), and then we phosphorylated AKT and total AKT were detected by Western blot. (D) Effect of Gβγ-interacting proteins on LPA-induced recruitment of Gβγ and downstream effectors to Rab11-positive endosomal compartments. HEK-293T cells transiently transfected with Gαi2 or Flag-PhLP1 were serum starved for 15 h followed by stimulation with 10 μM LPA during 30 min or left unstimulated (NS). The presence of Gβ1, PI3-kinase-γ, total or phosphorylated AKT, and the other indicated proteins in the endosomal fraction or total lysates was determined by Western blotting using specific antibodies. To assess the phosphorylation of AKT in samples with similar amounts of endosomal AKT, the sample obtained from control cells stimulated with 10 μM LPA was diluted as indicated, and then phosphorylated AKT and total AKT were detect Western blot. The expression of transfected Gαi2 or PhLP1 in total lysates was demonstrated by Western blot.
Figure 7.
Expression of dominant-negative Rab11a, Gαi2, or PTX treatment prevents the antiapoptotic and proliferative effect of LPA. (A) Antiapoptotic effect of 10 μM LPA was assessed by Annexin V binding in HEK-293T cells transfected with wild-type, constitutively active, or dominant-negative GFP-tagged mutants of Rab11a (Rab11a WT, Rab11a Q70L, or GFP-Rab11a S25N, respectively), Gαi2, or PTX treatment. NS, nonstimulated cells. The fraction of apoptotic cells in vector-transfected nonstimulated cells was normalized to 100%. Error bars represent the means ± SEM of three independent experiments (analysis of variance and Student-Newman-Keuls test). *p < 0.001 and **p < 0.05 compared with nonstimulated cells. (B) Proliferative effect of 10 μM LPA was assessed by BrdU labeling in HEK-293T cells transfected with wild-type, constitutively active, or dominant-negative GFP-tagged mutants of Rab11a (WT, Q70L, and S25N, respectively), Gαi2, or PTX treatment. NS, nonstimulated cells. Error bars represent the means ± SEM of four independent experiments done by triplicate (analysis of variance and Student-Newman-Keuls test). *p < 0.01 and **p < 0.001 compared with nonstimulated cells. (C) Effect of LPA and PTX on the recruitment to endosomes and phosphorylation of GSK3-β. Total cell lysates and endosomal fractions from samples shown in the Figure 6C were used to detect the presence and endosomal phosphorylation of GSK3-β by using specific antibodies. NS, nonstimulated cells. (D) Rab11 knockdown interferes with the antiapoptotic effect of LPA. Antiapoptotic effect of 10 μM LPA was assessed by Annexin V binding in HEK-293T cells transfected with Rab11 shRNA. NS, nonstimulated cells. Error bars represent the means ± SEM of four independent experiments (analysis of variance and Student-Newman-Keuls test). *p < 0.05 compared with vector-transfected cells. (E) Rab11 knockdown does not globally disrupt LPA-dependent signaling pathways. HEK-293T cells transfected with Rab11 shRNA were serum starved and stimulated with 10 μM LPA at indicated times. Phosphorylation of AKT and ERK in total cell lysates was detected by Western blotting using phospho-specific antibodies. The expression of AKT, ERK, Rab11, and Rab5 was also detected by Western blot as indicated. A representative blot is presented.
Figure 8.
Model depicting the redistribution and endosomal signaling of Gβγ. GPCR activation induces the dissociation of heterotrimeric G proteins into Gα and Gβγ subunits. The liberated Gβγ heterodimer activates its effectors such as PI3-kinase-γ at the plasma membrane, leading to the activation of AKT (1). Gβγ also interacts with Rab11a, promoting Gβγ trafficking to early and slowly recycling endosomes (as defined by the presence of Rab11a) (2). The assembly of a signaling complex occurs at endosomes after recruitment of PI3-kinase-γ followed by activation of AKT (steps 3 and 4). A fraction of AKT is associated with this endosomal fraction even in nonstimulated cells, but the association of this protein is further increased in response to receptor stimulation leading to the phosphorylation of endosomal AKT (5). GSK-3-β is recruited in response to receptor stimulation (step 6). Inhibition of PI3-kinase by Wm prevents the association of PI3-kinase-γ to endosomes and the phosphorylation of AKT. To complete the cycle, presumably Gβγ is recycled to the plasma membrane to start a new cycle of signaling (7).
Similar articles
- Recycling and Endosomal Sorting of Protease-activated Receptor-1 Is Distinctly Regulated by Rab11A and Rab11B Proteins.
Grimsey NJ, Coronel LJ, Cordova IC, Trejo J. Grimsey NJ, et al. J Biol Chem. 2016 Jan 29;291(5):2223-36. doi: 10.1074/jbc.M115.702993. Epub 2015 Dec 3. J Biol Chem. 2016. PMID: 26635365 Free PMC article. - Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases.
Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Roosterman D, et al. J Biol Chem. 2004 Jul 16;279(29):30670-9. doi: 10.1074/jbc.M402479200. Epub 2004 May 5. J Biol Chem. 2004. PMID: 15128739 - G-protein βγ subunits as multi-functional scaffolds and transducers in G-protein-coupled receptor signaling.
Smrcka AV, Fisher I. Smrcka AV, et al. Cell Mol Life Sci. 2019 Nov;76(22):4447-4459. doi: 10.1007/s00018-019-03275-2. Epub 2019 Aug 21. Cell Mol Life Sci. 2019. PMID: 31435698 Free PMC article. Review. - Gβγ Pathways in Cell Polarity and Migration Linked to Oncogenic GPCR Signaling: Potential Relevance in Tumor Microenvironment.
Vázquez-Prado J, Bracho-Valdés I, Cervantes-Villagrana RD, Reyes-Cruz G. Vázquez-Prado J, et al. Mol Pharmacol. 2016 Nov;90(5):573-586. doi: 10.1124/mol.116.105338. Epub 2016 Sep 16. Mol Pharmacol. 2016. PMID: 27638873 Review.
Cited by
- The orchestrated signaling by PI3Kα and PTEN at the membrane interface.
Kotzampasi DM, Premeti K, Papafotika A, Syropoulou V, Christoforidis S, Cournia Z, Leondaritis G. Kotzampasi DM, et al. Comput Struct Biotechnol J. 2022 Oct 7;20:5607-5621. doi: 10.1016/j.csbj.2022.10.007. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36284707 Free PMC article. Review. - All G protein βγ complexes are capable of translocation on receptor activation.
Ajith Karunarathne WK, O'Neill PR, Martinez-Espinosa PL, Kalyanaraman V, Gautam N. Ajith Karunarathne WK, et al. Biochem Biophys Res Commun. 2012 May 11;421(3):605-11. doi: 10.1016/j.bbrc.2012.04.054. Epub 2012 Apr 19. Biochem Biophys Res Commun. 2012. PMID: 22538369 Free PMC article. - Polarity mediates asymmetric trafficking of the Gbeta heterotrimeric G-protein subunit GPB-1 in C. elegans embryos.
Thyagarajan K, Afshar K, Gönczy P. Thyagarajan K, et al. Development. 2011 Jul;138(13):2773-82. doi: 10.1242/dev.063354. Development. 2011. PMID: 21652650 Free PMC article. - G-protein subunit gamma-4 expression has potential for detection, prediction and therapeutic targeting in liver metastasis of gastric cancer.
Tanaka H, Kanda M, Miwa T, Umeda S, Sawaki K, Tanaka C, Kobayashi D, Hayashi M, Yamada S, Nakayama G, Koike M, Kodera Y. Tanaka H, et al. Br J Cancer. 2021 Jul;125(2):220-228. doi: 10.1038/s41416-021-01366-1. Epub 2021 Apr 14. Br J Cancer. 2021. PMID: 33854208 Free PMC article.
References
- Ahn S., Shenoy S. K., Wei H., Lefkowitz R. J. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279:35518–35525. - PubMed
- Albert P. R., Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. - PubMed
- Barber M. A., Donald S., Thelen S., Anderson K. E., Thelen M., Welch H. C. Membrane translocation of P-Rex1 is mediated by G protein beta gamma subunits and phosphoinositide 3-kinase. J. Biol. Chem. 2007;282:29967–29976. - PubMed
- Bourne H. R. How receptors talk to trimeric G proteins. Curr. Opin. Cell Biol. 1997;9:134–142. - PubMed
- Bourne H. R. G-proteins and GPCrs: from the beginning. Ernst Schering Found. Symp. Proc. 2006:1–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous