p38 alpha MAPK inhibits JNK activation and collaborates with IkappaB kinase 2 to prevent endotoxin-induced liver failure - PubMed (original) (raw)
p38 alpha MAPK inhibits JNK activation and collaborates with IkappaB kinase 2 to prevent endotoxin-induced liver failure
Jan Heinrichsdorff et al. EMBO Rep. 2008 Oct.
Abstract
Activation of c-Jun amino-terminal kinase (JNK) facilitates tumour necrosis factor (TNF)-induced cell death. The p38 mitogen-activated protein kinase pathway is induced by TNF stimulation, but it has not been implicated in TNF-induced cell death. Here, we show that hepatocyte-specific ablation of p38alpha in mice results in excessive activation of JNK in the liver after in vivo challenge with bacterial lipopolysaccharide (LPS). Despite increased JNK activity, p38alpha-deficient hepatocytes were not sensitive to LPS/TNF toxicity showing that JNK activation was not sufficient to mediate TNF-induced liver damage. By contrast, LPS injection caused liver failure in mice lacking both p38alpha and IkappaB kinase 2 (IKK2) in hepatocytes. Therefore, when combined with partial nuclear factor-kappaB inhibition, p38alpha deficiency sensitizes the liver to cytokine-induced damage. Collectively, these results reveal a new function of p38alpha in collaborating with IKK2 to protect the liver from LPS/TNF-induced failure by controlling JNK activation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
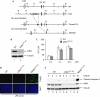
p38αLPC-KO mice are not sensitive to LPS-induced liver failure. (A) Schematic description of the targeting strategy for the generation of mice with loxP-flanked p38α alleles. Filled boxes indicate the loxP-flanked exons 2 and 3 (E2, E3), which include the ATP-binding site of the kinase domain. B, _Bam_HI; H, _Hin_dIII. Black arrowheads indicate loxP sites; white arrows indicate FLP recombinase target (Frt) sites. (B) Immunoblot analysis of expression of p38α in liver extracts from wild-type (WT) and p38αLPC-KO mice. (C–E) Assessment of liver damage in p38αLPC-KO and control mice after LPS injection. (C) Levels of free circulating alanine aminotransferases (ALTs) were measured in the serum of p38αLPC-KO and control mice at the indicated time points after LPS injection. Error bars denote s.e.m. (_n_=4). (D) Detection of apoptotic cells by TUNEL assay in livers from WT, p38αLPC-KO and NEMOLPC-KO (used as a positive control) mice 10 h after LPS injection. (E) Immunoblot analysis of caspase 3 activation using an antibody that specifically detects the cleaved form. DAPI, 4,6-diamidino-2-phenylindole; LPS, lipopolysaccharide; TUNEL, TdT-mediated dUTP nick end labelling.
Figure 2
Increased activation of JNK in the liver of p38αLPC-KO mice by LPS injection. (A) Phosphorylation of JNK was assessed in liver extracts from wild-type (WT) and p38αLPC-KO mice by immunoblot analysis using phospho-JNK-specific antibodies (upper panel). JNK immunoblot acts as a loading control. (B) Immunoblot analysis of phosphorylation of JNK in NEMOLPC-KO and control (WT) mice. (C) Immunoblot analysis with antibodies recognizing phosphorylated c-Jun (upper panel), total c-Jun (middle panel) and tubulin (lower panel) as a loading control. (D) The levels of c-FLIP(L) in WT, p38αLPC-KO and NEMOLPC-KO mice were analysed by immunoblot at the indicated time points after LPS injection. JNK, c-Jun amino-terminal kinase; LPS, lipopolysaccharide.
Figure 3
Hyperphosphorylation of JNK in the liver of LPS-injected p38αLPC-KO correlates with increased activation of MKK4. (A) Wild-type (WT; lanes 1–3), p38αLPC-KO (lanes 4–6) and p38αLPC-KO mice that had been pretreated with the antioxidant compound BHA (lanes 7 and 8) were injected with LPS and killed at the indicated time points. Immunoblot analysis was carried out on extracts of liver protein using antibodies against phosphorylated JNK or total JNK proteins. (B) Immunoblot analysis of the phosphorylation status of the JNK-activating kinases MKK4 and MKK7 in the liver extracts from WT and p38αLPC-KO mice at the indicated time points after LPS injection. Antibodies recognizing phosphorylated or total MKK7 or MKK4 were used. Tubulin acts as a loading control. n.s. indicates a nonspecific band. (C) Immunoblot analysis using an antibody detecting the phosphorylated forms of MKK3 and MKK6 (upper panel). Immunoblots with antibodies against total MKK3 and tubulin (lower panels) act as a loading control. BHA, butylated hydroxyanisole; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; MKK, mitogen-activated protein kinase kinase.
Figure 4
p38α collaborates with IKK2 to protect the liver from LPS-induced toxicity. (A) Immunoblot analysis for the expression of IKK2 and p38α in the extracts of liver protein from wild-type (WT), p38αLPC-KO and p38α/IKK2LPC-KO mice. (B) Levels of free circulating ALT were measured in IKK2LPC-KO, p38α/IKK2LPC-KO and control (WT) mice before and 10 h after LPS injection. Error bars denote s.e.m. *Statistical significance by Student's _t-_test with P<0.05 relative to control (_n_=4). Mean values are depicted above each column. (C) Detection of apoptotic cells by TUNEL assay in liver sections from WT, IKK2LPC-KO and p38α/IKK2LPC-KO mice 10 h after LPS injection. (D) Immunoblot analysis of caspase 3 activation using antibodies that specifically detect total caspase 3 (top panel) or the cleaved form of caspase 3 (middle panel) in liver extracts from mice with the indicated genotypes 10 h after LPS injection. NEMOLPC-KO mice were used as a positive control. Tubulin acts as a loading control. (E) The levels of c-FLIP(L) were measured by immunoblot analysis in livers from WT, p38αLPC-KO and p38α/IKK2LPC-KO mice at the indicated time points after LPS injection. Each lane represents an individual mouse. ALT, alanine aminotransferase; DAPI, 4,6-diamidino-2-phenylindole; IKK, IκB kinase; LPS, lipopolysaccharide; TUNEL, TdT-mediated dUTP nick end labelling.
Figure 5
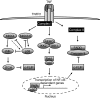
p38α collaborates with IKK2 to prevent liver failure in response to in vivo LPS/TNF challenge. TNF binding to TNFRI induces the activation of NF-κB and MAPK pathways, but it can also induce cell death through the activation of caspase 8. Activation of NF-κB protects cells from TNF-induced cell death by inducing the expression of anti-apoptotic proteins such as c-FLIP. Activation of JNK induces the E3 ubiquitin ligase ITCH to ubiquitinate c-FLIP leading to its degradation. Lack of p38α in hepatocytes leads to hyperactivation of MKK3/6, MKK4 and JNK on in vivo LPS challenge. The increased sustained activation of JNK is not sufficient to induce cell death in the p38α-deficient liver. When p38α ablation is combined with moderate inhibition of NF-κB, achieved by hepatocyte-restricted IKK2 ablation, in vivo LPS challenge results in increased degradation of c-FLIP and liver damage through caspase 8-mediated hepatocyte apoptosis. IKK, IκB kinase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; TNF, tumour necrosis factor.
Similar articles
- Inhibition of endocytosis exacerbates TNF-α-induced endothelial dysfunction via enhanced JNK and p38 activation.
Choi H, Nguyen HN, Lamb FS. Choi H, et al. Am J Physiol Heart Circ Physiol. 2014 Apr 15;306(8):H1154-63. doi: 10.1152/ajpheart.00885.2013. Epub 2014 Feb 21. Am J Physiol Heart Circ Physiol. 2014. PMID: 24561862 - IKappaB-kinase/nuclear factor-kappaB signaling prevents thermal injury-induced gut damage by inhibiting c-Jun NH2-terminal kinase activation.
Chen LW, Chen PH, Chang WJ, Wang JS, Karin M, Hsu CM. Chen LW, et al. Crit Care Med. 2007 May;35(5):1332-40. doi: 10.1097/01.CCM.0000261891.30360.F0. Crit Care Med. 2007. PMID: 17414734 - Estrogen attenuates lipopolysaccharide-induced nitric oxide production in macrophages partially via the nongenomic pathway.
Liu L, Wang Z. Liu L, et al. Cell Immunol. 2013 Nov-Dec;286(1-2):53-8. doi: 10.1016/j.cellimm.2013.11.004. Epub 2013 Nov 21. Cell Immunol. 2013. PMID: 24321566 - Mechanisms of crosstalk between TNF-induced NF-kappaB and JNK activation in hepatocytes.
Wullaert A, Heyninck K, Beyaert R. Wullaert A, et al. Biochem Pharmacol. 2006 Oct 30;72(9):1090-101. doi: 10.1016/j.bcp.2006.07.003. Epub 2006 Aug 24. Biochem Pharmacol. 2006. PMID: 16934229 Review.
Cited by
- Neuronal MAP kinase p38α inhibits c-Jun N-terminal kinase to modulate anxiety-related behaviour.
Stefanoska K, Bertz J, Volkerling AM, van der Hoven J, Ittner LM, Ittner A. Stefanoska K, et al. Sci Rep. 2018 Sep 24;8(1):14296. doi: 10.1038/s41598-018-32592-y. Sci Rep. 2018. PMID: 30250211 Free PMC article. - Targeting innate immunity protein kinase signalling in inflammation.
Gaestel M, Kotlyarov A, Kracht M. Gaestel M, et al. Nat Rev Drug Discov. 2009 Jun;8(6):480-99. doi: 10.1038/nrd2829. Nat Rev Drug Discov. 2009. PMID: 19483709 Review. - Systematic analysis of the MAPK signaling network reveals MAP3K-driven control of cell fate.
Peterson AF, Ingram K, Huang EJ, Parksong J, McKenney C, Bever GS, Regot S. Peterson AF, et al. Cell Syst. 2022 Nov 16;13(11):885-894.e4. doi: 10.1016/j.cels.2022.10.003. Epub 2022 Nov 9. Cell Syst. 2022. PMID: 36356576 Free PMC article. - MAPK signaling in inflammation-associated cancer development.
Huang P, Han J, Hui L. Huang P, et al. Protein Cell. 2010 Mar;1(3):218-26. doi: 10.1007/s13238-010-0019-9. Epub 2010 Feb 23. Protein Cell. 2010. PMID: 21203968 Free PMC article. Review. - Real-time imaging reveals that P2Y2 and P2Y12 receptor agonists are not chemoattractants and macrophage chemotaxis to complement C5a is phosphatidylinositol 3-kinase (PI3K)- and p38 mitogen-activated protein kinase (MAPK)-independent.
Isfort K, Ebert F, Bornhorst J, Sargin S, Kardakaris R, Pasparakis M, Bähler M, Schwerdtle T, Schwab A, Hanley PJ. Isfort K, et al. J Biol Chem. 2011 Dec 30;286(52):44776-87. doi: 10.1074/jbc.M111.289793. Epub 2011 Nov 4. J Biol Chem. 2011. PMID: 22057273 Free PMC article.
References
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR (2000) Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol Cell 6: 109–116 - PubMed
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M (2006) The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIP(L) turnover. Cell 124: 601–613 - PubMed
- Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ (1995) Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science 267: 682–685 - PubMed
- Dong C, Davis RJ, Flavell RA (2002) MAP kinases in the immune response. Annu Rev Immunol 20: 55–72 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous