The activity of the amphipathic peptide delta-lysin correlates with phospholipid acyl chain structure and bilayer elastic properties - PubMed (original) (raw)
The activity of the amphipathic peptide delta-lysin correlates with phospholipid acyl chain structure and bilayer elastic properties
Antje Pokorny et al. Biophys J. 2008.
Abstract
Release of lipid vesicle content induced by the amphipathic peptide delta-lysin was investigated as a function of lipid acyl chain length and degree of unsaturation for a series of phosphatidylcholines. Dye efflux and peptide binding were examined for three homologous lipid series: di-monounsaturated, di-polyunsaturated, and asymmetric phosphatidylcholines, with one saturated and one monounsaturated acyl chain. Except for the third series, peptide activity correlated with the first moment of the lateral pressure profile, which is a function of lipid acyl chain structure. In vesicles composed of asymmetric phosphatidylcholines, peptide binding and dye efflux are enhanced compared to symmetric, unsaturated lipids with similar pressure profiles. We attribute this to the entropically more favorable interaction of delta-lysin with partially saturated phospholipids. We find that lipid acyl chain structure has a major impact on the activity of delta-lysin and is likely to be an important factor contributing to the target specificity of amphipathic peptides.
Figures
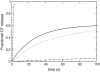
FIGURE 1
CF efflux induced by _δ_-lysin (0.5 _μ_M) for a series of di-monounsaturated PCs: di14:1PC (dashed line), di16:1PC (solid line), di18:1PC (dotted line), and di20:1PC (_dashed_-dotted line). The lipid concentration in all experiments was 50 _μ_M.
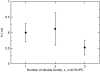
FIGURE 2
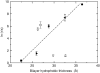
The average time constant (τ) for CF release induced by _δ_-lysin from lipid vesicles as a function of the number of double bonds in di18:_x_PC, where x represents the number of double bonds. Each error bar represents the standard deviation of a minimum of five individual traces. In the kinetic experiments, the peptide concentration was 0.5 _μ_M and the lipid concentration was 50 _μ_M.
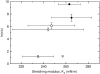
FIGURE 3
The average time constant (τ) for CF release induced by _δ_-lysin as a function of the stretching modulus, _K_A, for POPC (∇), SOPC (Δ), di18:3PC (◊), di18:2PC (□), di18:1PC (♦), and di22:1PC (•). Each vertical error bar represents the standard deviation of a minimum of five individual kinetic traces. The peptide concentration in the dye release experiments was 0.5 _μ_M and the lipid concentration was 50 _μ_M. The values for _K_A and their standard deviations were taken from Rawicz et al. (39).
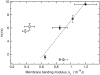
FIGURE 4
The average time constant for CF release induced by _δ_-lysin from lipid vesicles as a function of the bending modulus, _k_c. Solid symbols correspond to the di-monounsaturated PCs di16:1PC (▸), di18:1PC (♦), di20:1PC (◂), and di22:1PC (•). Open square symbols correspond to the di-polyunsaturated PCs di18:3PC (◊) and di18:2PC (□). Open triangular symbols correspond to POPC (∇) and SOPC (Δ). Each vertical error bar represents the standard deviation of a minimum of five individual kinetic traces. The dashed line is a linear fit to the data for the di-monounsaturated PCs (solid symbols). The peptide concentration in the dye release experiments was 0.5 _μ_M and the lipid concentration was 50 _μ_M. The values for _k_c and their standard deviations were taken from Rawicz et al. (39) and Kučerka et al. (52).
FIGURE 5
The average time constant for CF release induced by _δ_-lysin from lipid vesicles as a function of hydrophobic thickness, h. Solid symbols correspond to the di-monounsaturated PCs di14:1PC (▪), di16:1PC (▸), di18:1PC (♦), di20:1PC (◂), and di22:1PC (•). Open square symbols correspond to the di-polyunsaturated PCs di18:3PC (◊) and di18:2PC (□). Open triangular symbols correspond to POPC (∇) and SOPC (Δ). Each error bar represents the standard deviation of a minimum of five individual kinetic traces. The dashed line is a linear fit to the data for the di-monounsaturated PCs (solid circles). The peptide concentration in the dye release experiments was 0.5 _μ_M and the lipid concentration was 50 _μ_M. The values for h were taken from Hinderliter et al. (64).
Similar articles
- Branched phospholipids render lipid vesicles more susceptible to membrane-active peptides.
Mitchell NJ, Seaton P, Pokorny A. Mitchell NJ, et al. Biochim Biophys Acta. 2016 May;1858(5):988-94. doi: 10.1016/j.bbamem.2015.10.014. Epub 2015 Oct 26. Biochim Biophys Acta. 2016. PMID: 26514602 Free PMC article. - Mechanism and kinetics of delta-lysin interaction with phospholipid vesicles.
Pokorny A, Birkbeck TH, Almeida PF. Pokorny A, et al. Biochemistry. 2002 Sep 10;41(36):11044-56. doi: 10.1021/bi020244r. Biochemistry. 2002. PMID: 12206677 - Permeabilization of raft-containing lipid vesicles by delta-lysin: a mechanism for cell sensitivity to cytotoxic peptides.
Pokorny A, Almeida PF. Pokorny A, et al. Biochemistry. 2005 Jul 12;44(27):9538-44. doi: 10.1021/bi0506371. Biochemistry. 2005. PMID: 15996108 - Structure and functional properties of diacylglycerols in membranes.
Goñi FM, Alonso A. Goñi FM, et al. Prog Lipid Res. 1999 Jan;38(1):1-48. doi: 10.1016/s0163-7827(98)00021-6. Prog Lipid Res. 1999. PMID: 10396601 Review. - GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery.
Li W, Nicol F, Szoka FC Jr. Li W, et al. Adv Drug Deliv Rev. 2004 Apr 23;56(7):967-85. doi: 10.1016/j.addr.2003.10.041. Adv Drug Deliv Rev. 2004. PMID: 15066755 Review.
Cited by
- Mechanisms of antimicrobial, cytolytic, and cell-penetrating peptides: from kinetics to thermodynamics.
Almeida PF, Pokorny A. Almeida PF, et al. Biochemistry. 2009 Sep 1;48(34):8083-93. doi: 10.1021/bi900914g. Biochemistry. 2009. PMID: 19655791 Free PMC article. - Branched phospholipids render lipid vesicles more susceptible to membrane-active peptides.
Mitchell NJ, Seaton P, Pokorny A. Mitchell NJ, et al. Biochim Biophys Acta. 2016 May;1858(5):988-94. doi: 10.1016/j.bbamem.2015.10.014. Epub 2015 Oct 26. Biochim Biophys Acta. 2016. PMID: 26514602 Free PMC article. - Expression and characterization of cecropinXJ, a bioactive antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in Escherichia coli.
Xia L, Zhang F, Liu Z, Ma J, Yang J. Xia L, et al. Exp Ther Med. 2013 Jun;5(6):1745-1751. doi: 10.3892/etm.2013.1056. Epub 2013 Apr 9. Exp Ther Med. 2013. PMID: 23837066 Free PMC article. - Detergent-like activity and alpha-helical structure of warnericin RK, an anti-Legionella peptide.
Verdon J, Falge M, Maier E, Bruhn H, Steinert M, Faber C, Benz R, Héchard Y. Verdon J, et al. Biophys J. 2009 Oct 7;97(7):1933-40. doi: 10.1016/j.bpj.2009.06.053. Biophys J. 2009. PMID: 19804724 Free PMC article. - What determines the activity of antimicrobial and cytolytic peptides in model membranes.
Clark KS, Svetlovics J, McKeown AN, Huskins L, Almeida PF. Clark KS, et al. Biochemistry. 2011 Sep 20;50(37):7919-32. doi: 10.1021/bi200873u. Epub 2011 Aug 26. Biochemistry. 2011. PMID: 21870782 Free PMC article.
References
- Nickel, W. 2003. The mystery of nonclassical protein secretion. A current view on cargo proteins and potential export routes. Eur. J. Biochem. 270:2109–2119. - PubMed
- Handbook of Cell-Penetrating Peptides, 2nd Ed. 2006. U. Langel, editor. CRC, Boca Raton, FL.
- Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature. 415:389–395. - PubMed
- Ehrenstein, G., and H. Lecar. 1977. Electrically gated ionic channels in lipid bilayers. Q. Rev. Biophys. 10:1–34. - PubMed
- Ludtke, S. J., K. He, W. T. Heller, T. A. Harroun, L. Yang, and H. W. Huang. 1996. Membrane pores induced by magainin. Biochemistry. 35:13723–13728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources