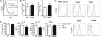TSLP acts on infiltrating effector T cells to drive allergic skin inflammation - PubMed (original) (raw)
TSLP acts on infiltrating effector T cells to drive allergic skin inflammation
Rui He et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Thymic stromal lymphopoietin (TSLP) is a cytokine expressed by epithelial cells, including keratinocytes, and is important in allergic inflammation. Allergic skin inflammation elicited by epicutaneous immunization of mice with ovalbumin (OVA), a potential model of atopic dermatitis, was severely impaired in TSLPR(-/-) mice, as evidenced by decreased infiltration of eosinophils and decreased local expression of T helper 2 (Th2) cytokines. However, secretion of Th2 cytokines by splenocytes from epicutaneous sensitized TSLPR(-/-) mice in response to OVA was normal. Skin dendritic cells from TSLPR(-/-) mice were normal in their ability to migrate to draining lymph nodes, express activation markers, and induce proliferation and Th2 cytokine production by naïve T cells. CD4(+) T cells from TSLPR(-/-) mice expressed the skin homing receptor E-selectin ligand normally, and homed to the skin normally, but failed to transfer allergic skin inflammation to WT recipients. TSLP enhanced Th2 cytokine secretion in vitro by targeting TSLPR on antigen specific T cells. Intradermal injection of anti-TSLP blocked the development of allergic skin inflammation after cutaneous antigen challenge of OVA immunized WT mice. These findings suggest that TSLP is essential for antigen driven Th2 cytokine secretion by skin infiltrating effector T cells and could be a therapeutic target in allergic skin inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
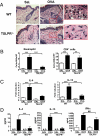
Fig. 1.
Decreased allergic skin inflammation in EC-sensitized TSLPR−/− mice. (A) H&E staining of sections from saline- and OVA-sensitized skin at ×200 magnification. Further magnification of the red bordered box shows the presence of multiple eosinophils (arrows). (B) Skin infiltration of eosinophils and CD4+ T cells. (C) Quantitative PCR analysis of mRNA levels of IL-4 and IL-13 in sensitized skin expressed as fold induction compared with saline-sensitized skin sites. (D) IL-4, IL-13, and IFN-γ secretion by cells from skin DLNs after stimulation for 4 days with OVA. Columns and error bars represent the mean ± SEM (n = 5–7 per group). *, P < 0.05, **, P < 0.01, ***, P < 0.0001. N.D., not detectable.
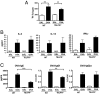
Fig. 2.
Systemic immune response in EC-sensitized TSLPR−/− mice. Proliferation (A) and secretion of IL-4, IL-13, and IFN-γ (B) by splenocytes from OVA EC-sensitized WT and TSLPR−/− mice after in vitro OVA stimulation. (C) Serum levels of OVA-specific IgE, IgG1, and IgG2a in EC-sensitized WT and TSLPR−/− mice. Columns and error bars represent the mean ± SEM (n = 6 per group), and similar results were obtained in two other independent experiments with 4–6 mice per group. *, P < 0.05, **, P < 0.01, ***, P < 0.0001.
Fig. 3.
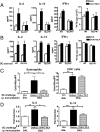
Skin DCs migrate, express activation markers, and present antigen normally in TSLPR−/− mice. Representative FACS analysis of percentage (A) and numbers (B) of CD11c+FITC+ cells in DLNs from WT and TSLPR−/− mice 24 h after FITC application to shaved skin. (C) Representative FACS analysis of expression of MHC class II, CD40, CD86, CD80, and OX40L on CD11c+FITC+ DCs of WT and TSLPR−/− mice. (D) Proliferation and secretion of IL-4, IL-13, and IFN-γ by DO11.10 CD4+ T cells stimulated with OVA323–339 peptide in the presence of skin-derived DCs from WT or TSLPR−/− mice. Data are representative of three experiments.
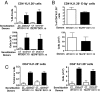
Fig. 4.
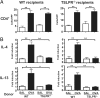
TSLPR−/− T cells express skin homing receptors and traffic to skin normally. Percentages and numbers of donor-derived CD4+KJ1–26+ cells (A) and CD4+KJ1–26+E-lig+ cells (B) in the DLNs of WT recipient mice challenged with OVA or control. (C) Percentages and numbers of donor-derived KJ1–26+ CD4+ T cells in the total cells extracted from the challenged ears of WT recipient mice. Columns and error bars represent the mean ± SEM (n = 3 per group).
Fig. 5.
CD4+ T cells from TSLPR−/− mice fail to transfer allergic skin inflammation. Number of infiltrating CD4+ cells (A) and expression of mRNA for IL-4 and IL-13 (B) in OVA-challenged skin of WT or TSLPR−/− recipient mice that received CD4+ T cells from EC-sensitized WT or TSLPR−/− donor mice. Columns and error bars represent the mean ± SEM of two experiments (n = 3–5 recipients per group). *, P < 0.05; **, P < 0.01.
Fig. 6.
TSLP amplifies Th2 cytokine secretion by engaging TSLPR on T cells. (A) Secretion of IL-4, IL-13, and IFN-γ and proliferation by splenocytes from EC-sensitized mice with OVA after in vitro OVA stimulation with or without TSLP. (B) Secretion of IL-4, IL-13, and IFN-γ and proliferation by DO11.10 CD4+ T cells stimulated with OVA323–339 peptide with or without TSLP in the presence of DCs from the spleens of WT or TSLPR−/− mice. Number of infiltrating eosinophils and CD4+ cells (C) and expression of mRNA for IL-4 and IL-13 (D) in OVA-challenged skin of i.p. immunized mice treated with neutralizing antibodies to TSLP or isotype control. Columns and error bars represent the mean ± SEM of 5 mice per group in A, C, and D, and mean ± SEM of four experiments in B. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Similar articles
- Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses.
Larson RP, Zimmerli SC, Comeau MR, Itano A, Omori M, Iseki M, Hauser C, Ziegler SF. Larson RP, et al. J Immunol. 2010 Mar 15;184(6):2974-84. doi: 10.4049/jimmunol.0803478. Epub 2010 Feb 19. J Immunol. 2010. PMID: 20173025 Free PMC article. - Early production of thymic stromal lymphopoietin precedes infiltration of dendritic cells expressing its receptor in allergen-induced late phase cutaneous responses in atopic subjects.
Corrigan CJ, Jayaratnam A, Wang Y, Liu Y, de Waal Malefyt R, Meng Q, Kay AB, Phipps S, Lee TH, Ying S. Corrigan CJ, et al. Allergy. 2009 Jul;64(7):1014-22. doi: 10.1111/j.1398-9995.2009.01947.x. Epub 2009 Feb 2. Allergy. 2009. PMID: 19187393 - Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory.
Wang Q, Du J, Zhu J, Yang X, Zhou B. Wang Q, et al. J Allergy Clin Immunol. 2015 Mar;135(3):781-91.e3. doi: 10.1016/j.jaci.2014.09.015. Epub 2014 Oct 24. J Allergy Clin Immunol. 2015. PMID: 25441291 Free PMC article. - Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate-Adaptive Immunity of Pediatric Asthma.
Lin SC, Cheng FY, Liu JJ, Ye YL. Lin SC, et al. Int J Mol Sci. 2018 Apr 18;19(4):1231. doi: 10.3390/ijms19041231. Int J Mol Sci. 2018. PMID: 29670037 Free PMC article. Review. - Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses.
Wang YH, Liu YJ. Wang YH, et al. Clin Exp Allergy. 2009 Jun;39(6):798-806. doi: 10.1111/j.1365-2222.2009.03241.x. Epub 2009 Apr 7. Clin Exp Allergy. 2009. PMID: 19400908 Free PMC article. Review.
Cited by
- Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function.
Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, Harris NL. Massacand JC, et al. Proc Natl Acad Sci U S A. 2009 Aug 18;106(33):13968-73. doi: 10.1073/pnas.0906367106. Epub 2009 Aug 4. Proc Natl Acad Sci U S A. 2009. PMID: 19666528 Free PMC article. - β-Caryophyllene Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis through the Downregulation of Mitogen-Activated Protein Kinase/EGR1/TSLP Signaling Axis.
Ahn SS, Yeo H, Jung E, Ou S, Lee YH, Lim Y, Shin SY. Ahn SS, et al. Int J Mol Sci. 2022 Nov 28;23(23):14861. doi: 10.3390/ijms232314861. Int J Mol Sci. 2022. PMID: 36499191 Free PMC article. - From Bench to Clinic: the Potential of Therapeutic Targeting of the IL-22 Signaling Pathway in Atopic Dermatitis.
Jin M, Yoon J. Jin M, et al. Immune Netw. 2018 Dec 19;18(6):e42. doi: 10.4110/in.2018.18.e42. eCollection 2018 Dec. Immune Netw. 2018. PMID: 30619628 Free PMC article. Review. - A modular view of cytokine networks in atopic dermatitis.
Carmi-Levy I, Homey B, Soumelis V. Carmi-Levy I, et al. Clin Rev Allergy Immunol. 2011 Dec;41(3):245-53. doi: 10.1007/s12016-010-8239-6. Clin Rev Allergy Immunol. 2011. PMID: 21222175 Review.
References
- van Reijsen FC, et al. Skin-derived aeroallergen-specific T-cell clones of Th2 phenotype in patients with atopic dermatitis. J Allergy Clin Immunol. 1992;90:184–193. - PubMed
- Holgate ST. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 2007;28:248–251. - PubMed
- Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: Inflammatory signals. Curr Allergy Asthma Rep. 2004;4:276–284. - PubMed
- Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01 AI040030/AI/NIAID NIH HHS/United States
- R01 AR047417/AR/NIAMS NIH HHS/United States
- R01 AR056113/AR/NIAMS NIH HHS/United States
- AR-047417/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials