Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli - PubMed (original) (raw)
Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli
Silvia A Purro et al. J Neurosci. 2008.
Abstract
Axon guidance and target-derived signals control axonal behavior by regulating the cytoskeleton through poorly defined mechanisms. In particular, how these signaling molecules regulate the growth and directionality of microtubules is not well understood. Here we examine the effect of Wnts on growth cone remodeling, a process that precedes synapse formation. Time-lapse recordings reveal that Wnt3a rapidly inhibits growth cone translocation while inducing growth cone enlargement. These changes in axonal behavior are associated with changes in the organization of microtubules. Time-lapse imaging of EB3-GFP (green fluorescent protein)-labeled microtubule plus-ends demonstrates that Wnt3a regulates microtubule directionality, resulting in microtubule looping, growth cone pausing, and remodeling. Analyses of Dishevelled-1 (Dvl1) mutant neurons demonstrate that Dvl1 is required for Wnt-mediated microtubule reorganization and axon remodeling. Wnt signaling directly affects the microtubule cytoskeleton by unexpectedly inducing adenomatous polyposis coli (APC) loss from microtubule plus-ends. Consistently, short hairpin RNA knockdown of APC mimics Wnt3a function. Together, our findings define APC as a key Wnt signaling target in the regulation of microtubule growth direction.
Figures
Figure 1.
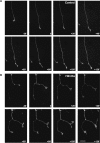
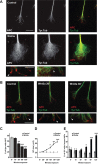
Wnt3a induces changes in axon behavior. A, Frames from a time-lapse recording illustrating the typical response of an axon growing in the presence of control media: steady advance is observed with only occasional pausing and no attempt of branching. Growth cone size does not significantly change. B, Frames illustrating the typical response to Wnt3a. After addition of Wnt3a at time point 0, the axon stops extending, the growth cone on the right attempts to branch, and both growth cones become enlarged. Scale bar, 20 μm. Wnt3a decreases the speed of growth cone advance within 20 min and increases growth cone size after 60 min (supplemental Video 1_A_,B, available at
as supplemental material).
Figure 2.
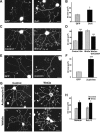
Wnt3a-induced axonal remodeling is mimicked by Dvl1 or β-catenin and inhibition of Gsk3β but does not require transcription. A, Expression of Dvl1 in DRG neurons induces growth cone enlargement (arrowheads), axon shortening, and branching compared with control EGP-expressing neurons. Scale bar, 30 μm. B, Quantification of growth cone (GC) size reveals a 30% increase in Dvl1-expressing cells compared with controls. C, Application of the Gsk3-specific pharmacological inhibitor Bio for 2 h mimics the effect of Wnt3a on axon remodeling (arrowheads). Scale bar, 30 μm. D, Quantification shows a significant increase in the size of growth cones (30%) in the presence of Bio. Expression of a constitutive form of Gsk3β (Gsk3βS9A) partially blocks the effect of Wnt3a on growth cone size. E, Expression of β-catenin-Arm (β-cat-Arm) induces growth cone enlargement in DRG neurons (arrowheads) compared with control EGP-expressing neurons. Scale bar, 30 μm. F, Quantification of growth cone size reveals a 60% increase in β-catenin-Arm-expressing cells compared with controls. G, Wnt3a induces axonal remodeling (arrowheads) in the presence of actinomycin D. Scale bar, 30 μm. H, Average growth cone size is significantly larger in DRGs exposed to both Wnt3a and actinomycin D (Act. D) than in controls and indistinguishable from neurons treated with both Wnt3a and vehicle. Values are mean ± SEM of three independent experiments. *p < 0.05; ***p < 0.001. n = 50.
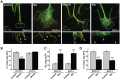
Figure 3.
Wnt3a and Dvl1 regulate the amount and organization of microtubules along axons and in growth cones. A, DRG neurons from wild-type and Dvl1 mutant mice were exposed to vehicle or Wnt3a for 2 h. In wild-type neurons, Wnt3a induces the formation of looped MTs at growth cones. In contrast, Dvl1 mutant neurons do not remodel, because their axons are long and terminate in small growth cones. Scale bars: top, 30 μm; bottom, 15 μm. B, Quantification shows that Wnt3a does not induce growth cone (GC) remodeling in Dvl1 mutant neurons. C, Graph illustrating that Wnt3a does not increase the percentage of growth cones with looped MTs in Dvl1 mutant neurons. Values are mean ± SEM of three independent experiments. **p < 0.01. n = 100.
Figure 4.
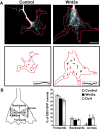
Wnt signaling induces the loss of directionality of microtubule growth. A, Kymographs of 10 consecutives frames of a control and Wnt3a-treated growth cone expressing EB3-GFP; the last frame was labeled in red to indicate directionality. In control neurons, most MTs splay as they enter the growth cone (red arrows). In the presence of Wnt3a, the direction of MT growth is altered as more MTs grow across the growth cone (black arrows). Insets, Traces of one representative microtubule from each growth cone. In Wnt3a-treated growth cones, MTs kink several times instead of growing with a persistent directionality. Scale bar, 15 μm. B, The diagram illustrates how the direction of EB3 comets was quantified. Quantification shows that Wnt3a and Dvl1 significantly increase the number of comets moving across the growth cone while decreasing the number moving forwards, toward the leading edge. The direction of EB3-GFP comets from at least 12 growth cones was analyzed per condition in four independent experiments. Values are mean ± SEM of at least three independent experiments. *p < 0.05. n = 200.
Figure 5.
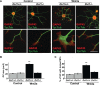
Wnt3a decreases the level of APC at microtubule plus-ends in the periphery of growth cones. A, In control DRG neurons, endogenous APC localizes along axonal MTs and at the plus-ends of splayed MTs at the leading edge of the growth cone. Application of Wnt3a for 2 h decreases the level of APC at MT plus-ends at the leading edge of the growth cone. Enlarged images of growth cones show the significant loss of APC at MT plus-ends (arrowheads) when neurons are exposed to Wnt3a. Scale bar, 15 μm. B, Time course experiment shows that APC is lost from MT tips by 30 min after Wnt3a exposure (arrowheads). C, Quantification shows that after 10 min of exposure to Wnt3a, the level of APC at the tips of MTs is similar to control untreated neurons, whereas after 30 min of application, a 25% loss of APC is observed at the tips of MTs, reaching up to a 55% after 120 min of Wnt exposure. D, Quantification shows a significant increase in growth cone (GC) size after 60 min on Wnt treatment. E, MT looping correlates with growth cone enlargement. Growth cones from DRG neurons in presence of Wnt3a exhibit more looped MT after 60 min of Wnt exposure. Values are mean ± SEM of at least three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. n = 100. Scale bars, 5 μm.
Figure 6.
Pharmacological inhibition of Gsk3 decreases the level of APC at microtubule plus-ends of DRG neurons from wild-type or Dvl1 mutant mice. A, In DRG neurons from wild-type mice, the Gsk3 inhibitor Bio induces the loss of APC from MT tips at the periphery of the growth cone (arrowheads). Similarly, DRG neurons from Dvl1 mutant mice show a decrease in the level of APC at the tips of MTs compared with control neurons (arrowheads). Scale bar, 15 μm. B, DRG neurons from Dvl1 mutant mice do not show a decrease in the number of MT plus-ends containing APC after Wnt3a treatment compared with untreated neurons. C, Quantification shows that Gsk3 inhibition with Bio increases growth cone (GC) size to the same extent on DRG neurons from wild-type or Dvl1 mutant mice. D, Quantification shows that the percentage of MT tips with APC per growth cone decreases similarly in wild-type or Dvl1 mutant DRG neurons when exposed to Bio. Values are mean ± SEM of at least three independent experiments. ***p < 0.001. n = 100.
Figure 7.
APC knockdown in wild-type or Dvl1 mutant DRG neurons induces the formation of looped microtubules and enlargement of growth cone size. A, DRG neurons coexpressing scrambled shRNA construct and EGFP have small growth cones like control neurons, whereas DRG neurons coexpressing an APC1 shRNA construct and EGFP have enlarged growth cones with looped MTs. Growth cones show the significant loss of APC at MT tips (arrowheads). Scale bars: top, 30 μm; bottom, 5 μm. See Results for quantifications of number of growth cones with looped MTs, size of growth cones, and percentage of MT tips containing APC. B, Dvl1 mutant DRG neurons exhibit enlarged growth cones with looped MT when coexpressing APC1 shRNA construct and EGFP. In contrast, Dvl1 mutant DRG neurons coexpressing scrambled shRNA construct and EGFP exhibit growth cones with comparable size to control neurons. Growth cones show the significant loss of APC at MT tips (arrowheads). Scale bars: top, 30 μm; bottom, 5 μm. See Results for quantification of percentage of MT tips with APC per growth cone. C, Model for Wnt signaling in the regulation of MT directionality. Activation of the Wnt-Dvl1 signaling pathway regulates APC localization on MT plus-ends at the periphery of the growth cone, resulting in MT looping and enlargement of growth cones.
Similar articles
- Wnt Signalling Promotes Actin Dynamics during Axon Remodelling through the Actin-Binding Protein Eps8.
Stamatakou E, Hoyos-Flight M, Salinas PC. Stamatakou E, et al. PLoS One. 2015 Aug 7;10(8):e0134976. doi: 10.1371/journal.pone.0134976. eCollection 2015. PLoS One. 2015. PMID: 26252776 Free PMC article. - NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC.
Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD. Zhou FQ, et al. Neuron. 2004 Jun 24;42(6):897-912. doi: 10.1016/j.neuron.2004.05.011. Neuron. 2004. PMID: 15207235 - c-Jun N-terminal kinase (JNK) cooperates with Gsk3beta to regulate Dishevelled-mediated microtubule stability.
Ciani L, Salinas PC. Ciani L, et al. BMC Cell Biol. 2007 Jul 3;8:27. doi: 10.1186/1471-2121-8-27. BMC Cell Biol. 2007. PMID: 17608927 Free PMC article. - [Dual-role regulations of canonical Wnt/beta-catenin signaling pathway].
Liu Y, Zhang CG, Zhou CY. Liu Y, et al. Beijing Da Xue Xue Bao Yi Xue Ban. 2010 Apr 18;42(2):238-42. Beijing Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20396373 Review. Chinese. - New steps in the Wnt/beta-catenin signal transduction pathway.
Sakanaka C, Sun TQ, Williams LT. Sakanaka C, et al. Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
Cited by
- Lithium alters the morphology of neurites regenerating from cultured adult spiral ganglion neurons.
Shah SM, Patel CH, Feng AS, Kollmar R. Shah SM, et al. Hear Res. 2013 Oct;304:137-44. doi: 10.1016/j.heares.2013.07.001. Epub 2013 Jul 12. Hear Res. 2013. PMID: 23856237 Free PMC article. - LRRK2 functions as a Wnt signaling scaffold, bridging cytosolic proteins and membrane-localized LRP6.
Berwick DC, Harvey K. Berwick DC, et al. Hum Mol Genet. 2012 Nov 15;21(22):4966-79. doi: 10.1093/hmg/dds342. Epub 2012 Aug 16. Hum Mol Genet. 2012. PMID: 22899650 Free PMC article. - Wnt Signalling Promotes Actin Dynamics during Axon Remodelling through the Actin-Binding Protein Eps8.
Stamatakou E, Hoyos-Flight M, Salinas PC. Stamatakou E, et al. PLoS One. 2015 Aug 7;10(8):e0134976. doi: 10.1371/journal.pone.0134976. eCollection 2015. PLoS One. 2015. PMID: 26252776 Free PMC article. - Dysregulation of multiple signaling pathways: A possible cause of cerebral palsy.
Upadhyay J, Ansari MN, Samad A, Sayana A. Upadhyay J, et al. Exp Biol Med (Maywood). 2022 May;247(9):779-787. doi: 10.1177/15353702221081022. Epub 2022 Mar 7. Exp Biol Med (Maywood). 2022. PMID: 35253451 Free PMC article. Review. - The Molecular Basis of Wnt/_β_-Catenin Signaling Pathways in Neurodegenerative Diseases.
Anand AA, Khan M, V M, Kar D. Anand AA, et al. Int J Cell Biol. 2023 Sep 21;2023:9296092. doi: 10.1155/2023/9296092. eCollection 2023. Int J Cell Biol. 2023. PMID: 37780577 Free PMC article. Review.
References
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. - PubMed
- Bienz M. The subcellular destinations of APC proteins. Nat Rev Mol Cell Biol. 2002;3:328–338. - PubMed
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. - PubMed
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- 074372/WT_/Wellcome Trust/United Kingdom
- 078764/WT_/Wellcome Trust/United Kingdom
- BBE0160061/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases