Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes - PubMed (original) (raw)
Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes
Susan M Nicholson-Dykstra et al. Cell Motil Cytoskeleton. 2008 Nov.
Abstract
The Arp2/3 complex-mediated assembly and protrusion of a branched actin network at the leading edge occurs during cell migration, although some studies suggest it is not essential. In order to test the role of Arp2/3 complex in leading edge protrusion, Swiss 3T3 fibroblasts and Jurkat T cells were depleted of Arp2 and evaluated for defects in cell morphology and spreading efficiency. Arp2-depleted fibroblasts exhibit severe defects in formation of sheet-like protrusions at early time points of cell spreading, with sheet-like protrusions limited to regions along the length of linear protrusions. However, Arp2-depleted cells are able to spread fully after extended times. Similarly, Arp2-depleted Jurkat T lymphocytes exhibit defects in spreading on anti-CD3. Interphase Jurkats in suspension are covered with large ruffle structures, whereas mitotic Jurkats are covered by finger-like linear protrusions. Arp2-depleted Jurkats exhibit defects in ruffle assembly but not in assembly of mitotic linear protrusions. Similarly, Arp2-depletion has no effect on the highly dynamic linear protrusion of another suspended lymphocyte line. We conclude that Arp2/3 complex plays a significant role in assembly of sheet-like protrusions, especially during early stages of cell spreading, but is not required for assembly of a variety of linear actin-based protrusions.
(c) 2008 Wiley-Liss, Inc.
Figures
Figure 1. Arp2 suppression in 3T3 Fibroblasts
(A) Western blot of Arp2 (upper) and actin (lower) levels in Control and Arp2-knockdown whole cell extracts. (B) Phase and immunofluorescent images depicting Control (upper) and Arp2-KD (lower) cell stained for Arp2 to demonstrate depletion of endogenous Arp2 in GFP+ anti-Arp2-shRNA transfected cells. (C–D) Phase (left) and fluorescent images of polymerized actin (center) and GFP (right) in Control (C) and Arp2-KD (D) cells 78 hours post-transfection. Cells were plated onto glass coverslips immediately after transfection. (E–F) Phase contrast images of Control (E) and Arp2-KD (F) cells plated onto glass coverslips directly after transfection, then observed 78 hours post transfection by live cell imaging to monitor peripheral protrusion dynamics. (G–H) Enlarged phase contrast time lapse image of regions from Control (G) and Arp2-KD (H) depicted by boxes in D,E. Time points indicate time in minutes during time course. Bar: B,C = 20 μm; D–G = 10 μm.
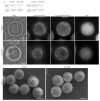
Figure 2. Fibroblast Spreading Assay
SEM (A–C) and immunofluorescent (D–F) staining of 3T3s replated after trypsinization on PLL-coated coverslips and fixed after 20 min (A, D), 60 min (B, E) or 24 hours (C, F). SEM images taken at 500x, bar = 50μm. D–F) Phase (left), polymerized actin (middle) and tubulin (right) staining. Polymerized actin labeled with rhodamine-phalloidin. Image levels adjusted independently to reflect localization and organization of polymerized actin and tubulin. (G,H) Fluorescent time-lapse images of 3T3s expressing 6His-GFP-Actin (G) or GFP-Arp3 (H) during replating time course on PLL-coated coverslips. Bar: D–F = 20 μm, G,H = 10 μm.
Figure 3. Spreading time course of Arp2-knockdown 3T3 fibroblasts on PLL
Images of phase and fluorescently stained F-Actin and tubulin in Empty Vector Control (A) and Arp2-knockdown cells after 20 min, 60 min, 120 min and 8 hours after trypsinization and replating on PLL-coated coverslips. Fluorescent levels separately adjusted to optimize view of F-actin and tubulin in each image. Bar: A–B = 10 μm. (C) Quantification of morphology of Empty Vector Control and Arp2-knockdown cells after 20 minutes of spreading on PLL-coated coverslips. (D) Quantification of number of Empty Vector Control and Arp2-knockdown cells adhered to coverslip and exhibiting a spread morphology similar to untransfected control cells (see Fig. 1).
Figure 4. Arp2-knockdown inhibits cell spreading and lamellipodial protrusion
(A–I) Phase contrast time lapse images of cells spreading on PLL-coated coverslips. (A) Untransfected control (A) and Arp2-knockdown (B) cells observed from 30–46 minutes after initiation of spreading assay. (C–E) Enlarged inset of peripheral lamellipodial protrusions (C) of control cell outlined in (A) and of lamellipodia-like protrusion along the length of pre-existing filopodia-like protrusions of Arp2-knockdown cells (D, solid box; E, hashed box) outlined in (B). Empty Vector Control (F) and Arp2-knockdown (G) cells observed 30–38 minutes after initiation of spreading assay. Enlarged inset of peripheral lamellipodial protrusions of control cell (H) outlined in (F) and of remaining lamellipodial protrusions of Arp2-knockdown cell (I) outlined in (G). Bar: A,B = 10μm; C–E = 5 μm; F,G = 10μm; H,I = 2 μm.
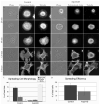
Figure 5. Arp2-depletion inhibits Jurkat ruffle formation
SEM (A) and fluorescent images of actin-rich ruffles (B) on the surface of Jurkat cells in suspension. SEM images of Control (C) and Arp2-KD (D) cells fixed in suspension 76–78 hours post-transfection. Bars: A–E = 2 μm. Cells were evaluated for the presence of ruffles, microvilli, blebs and bald surfaces (E).
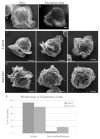
Figure 6. The microvilli of Arp2-depleted Jurkats are not a result of mitotic arrest
SEM (A) and fluorescent (B) images of untransfected Jurkat cells demonstrating presence of occasional microvillar-covered cells, clearly seen by low-magnification SEM images (A) and by imaging the apical surface of cells stained for polymerized actin (B). Microvilli-covered cells are outlined by boxes. (C,D) SEM (upper) and immunfluorescent images (lower) of Untreated Control cells (C) and Nocodazole-blocked cells 45 minutes post-washout (D). Fluorescent images include rhodamine-phalloidin stained actin on dorsal surface (upper), anti-tubulin stained microtubules (middle) and DAPI-stained DNA (lower). (E) Quantification of surface morphology (by SEM analysis) of untreated and post-nocodazole washout cells. Cells were evaluated for presence of ruffles, microvilli, blebs or bald surfaces. (F) Mitotic Index of control and 45 min post-nocodazole washout cells. Immunofluorescent images were evaluated for total number of interphase and mitotic cells in general population. (G) Mitotic Index of Control and Arp2-depleted cells 76 hours after transfection. Quantification presented in F,G is from a single experiment that is representative of multiple nocodazole block and release trials.
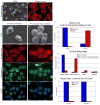
Figure 7. Arp2-depletion has no effect on microvillar formation in 300.19 cells
Western blot analysis of Control and Arp2-KD 300.19 cells (A) stained with anti-Arp2. (B,C) Phase (left) and fluorescent images of the actin-rich puncta and the peripheral band of actin of GFP+ Control (B) and Arp2-KD (C) cells. SEM images of Control (D) and Arp2-KD (E) cells fixed in suspension. Bar: B–E = 5 μm.
Similar articles
- Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts.
Mingle LA, Okuhama NN, Shi J, Singer RH, Condeelis J, Liu G. Mingle LA, et al. J Cell Sci. 2005 Jun 1;118(Pt 11):2425-33. doi: 10.1242/jcs.02371. J Cell Sci. 2005. PMID: 15923655 Free PMC article. - Mis-localization of Arp2 mRNA impairs persistence of directional cell migration.
Liao G, Simone B, Liu G. Liao G, et al. Exp Cell Res. 2011 Apr 1;317(6):812-22. doi: 10.1016/j.yexcr.2010.12.002. Epub 2010 Dec 10. Exp Cell Res. 2011. PMID: 21146522 Free PMC article. - Arp2/3 complex activity in filopodia of spreading cells.
Johnston SA, Bramble JP, Yeung CL, Mendes PM, Machesky LM. Johnston SA, et al. BMC Cell Biol. 2008 Dec 9;9:65. doi: 10.1186/1471-2121-9-65. BMC Cell Biol. 2008. PMID: 19068115 Free PMC article. - Does self-organized criticality drive leading edge protrusion?
Anderson KL, Swift MF, Hanein D, Volkmann N. Anderson KL, et al. Biophys Rev. 2018 Dec;10(6):1571-1575. doi: 10.1007/s12551-018-0484-6. Epub 2018 Nov 17. Biophys Rev. 2018. PMID: 30448941 Free PMC article. Review. - MyTH4-FERM myosins in the assembly and maintenance of actin-based protrusions.
Weck ML, Grega-Larson NE, Tyska MJ. Weck ML, et al. Curr Opin Cell Biol. 2017 Feb;44:68-78. doi: 10.1016/j.ceb.2016.10.002. Epub 2016 Nov 9. Curr Opin Cell Biol. 2017. PMID: 27836411 Free PMC article. Review.
Cited by
- Actin dynamics in cell migration.
Schaks M, Giannone G, Rottner K. Schaks M, et al. Essays Biochem. 2019 Oct 31;63(5):483-495. doi: 10.1042/EBC20190015. Essays Biochem. 2019. PMID: 31551324 Free PMC article. Review. - INF2 is an endoplasmic reticulum-associated formin protein.
Chhabra ES, Ramabhadran V, Gerber SA, Higgs HN. Chhabra ES, et al. J Cell Sci. 2009 May 1;122(Pt 9):1430-40. doi: 10.1242/jcs.040691. Epub 2009 Apr 14. J Cell Sci. 2009. PMID: 19366733 Free PMC article. - Loss of ARPC1B impairs cytotoxic T lymphocyte maintenance and cytolytic activity.
Randzavola LO, Strege K, Juzans M, Asano Y, Stinchcombe JC, Gawden-Bone CM, Seaman MN, Kuijpers TW, Griffiths GM. Randzavola LO, et al. J Clin Invest. 2019 Dec 2;129(12):5600-5614. doi: 10.1172/JCI129388. J Clin Invest. 2019. PMID: 31710310 Free PMC article. - The Actin Regulators Involved in the Function and Related Diseases of Lymphocytes.
Sun J, Zhong X, Fu X, Miller H, Lee P, Yu B, Liu C. Sun J, et al. Front Immunol. 2022 Mar 16;13:799309. doi: 10.3389/fimmu.2022.799309. eCollection 2022. Front Immunol. 2022. PMID: 35371070 Free PMC article. Review. - Nano-scale actin-network characterization of fibroblast cells lacking functional Arp2/3 complex.
Anderson KL, Page C, Swift MF, Suraneni P, Janssen MEW, Pollard TD, Li R, Volkmann N, Hanein D. Anderson KL, et al. J Struct Biol. 2017 Mar;197(3):312-321. doi: 10.1016/j.jsb.2016.12.010. Epub 2016 Dec 21. J Struct Biol. 2017. PMID: 28013022 Free PMC article.
References
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp Cell Res. 1970a;59(3):393–398. - PubMed
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. II. “Ruffling”. Exp Cell Res. 1970b;60(3):437–444. - PubMed
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. III. Movements of particles on the dorsal surface of the leading lamella. Exp Cell Res. 1970c;62(2):389–398. - PubMed
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67(2):359–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources