Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach - PubMed (original) (raw)
Randomized Controlled Trial
. 2008 Aug 14;14(30):4753-62.
doi: 10.3748/wjg.14.4753.
Affiliations
- PMID: 18720535
- PMCID: PMC2739336
- DOI: 10.3748/wjg.14.4753
Randomized Controlled Trial
Prevention of hepatotoxicity due to anti tuberculosis treatment: a novel integrative approach
Meghna R Adhvaryu et al. World J Gastroenterol. 2008.
Abstract
Aim: To evaluate the ability of Curcuma longa (CL) and Tinospora cordifolia (TC) formulation to prevent anti-tuberculosis (TB) treatment (ATT) induced hepatotoxicity.
Methods: Patients with active TB diagnosis were randomized to a drug control group and a trial group on drugs plus an herbal formulation. Isoniazid, rifampicin, pyrazinamide and ethambutol for first 2 mo followed by continuation phase therapy excluding Pyrazinamide for 4 mo comprised the anti-tuberculous treatment. Curcumin enriched (25%) CL and a hydro-ethanolic extract enriched (50%) TC 1 g each divided in two doses comprised the herbal adjuvant. Hemogram, bilirubin and liver enzymes were tested initially and monthly till the end of study to evaluate the result.
Results: Incidence and severity of hepatotoxicity was significantly lower in trial group (incidence: 27/192 vs 2/316, P<0.0001). Mean aspartate transaminase (AST) (195.93+/-108.74 vs 85+/-4.24, P<0.0001), alanine transaminase (ALT) (75.74+/-26.54 vs 41+/-1.41, P<0.0001) and serum bilirubin (5.4+/-3.38 vs 1.5+/-0.42, P<0.0001). A lesser sputum positivity ratio at the end of 4 wk (10/67 vs 4/137, P=0.0068) and decreased incidence of poorly resolved parenchymal lesion at the end of the treatment (9/152 vs 2/278, P=0.0037) was observed. Improved patient compliance was indicated by nil drop-out in trial vs 10/192 in control group (P<0.0001).
Conclusion: The herbal formulation prevented hepatotoxicity significantly and improved the disease outcome as well as patient compliance without any toxicity or side effects.
Figures
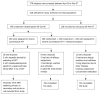
Figure 1
Study design and enrollment and follow-up of patients.
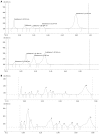
Figure 2
A: HPTLC finger print of formulation of Curcuma longa. Sample was extracted in MeOH and Solvent system for Mobile phase used was Chloroform: MeOH (99.5: 0.5). Note: Before loading the material on TLC plate, it was impregnated with di NaHPO4 (anhydrous)-1.25 g dissolved in 25 mL double distilled water (DDW). B: HPTLC finger print of formulation of Tinospora cordifolia. Sample was extracted in-MeOH and NH4OH: MeOH (9:1) and solvent system for mobile phase used was -Chloroform: MeOH (8:2).
Similar articles
- Hepatotoxicity due to rifampicin, isoniazid and pyrazinamide in patients with tuberculosis: is anti-HCV a risk factor?
Nader LA, de Mattos AA, Picon PD, Bassanesi SL, De Mattos AZ, Pineiro Rodriguez M. Nader LA, et al. Ann Hepatol. 2010 Jan-Mar;9(1):70-4. Ann Hepatol. 2010. PMID: 20308724 - Inactive hepatitis B surface antigen carrier state and hepatotoxicity during antituberculosis chemotherapy.
Lee BH, Koh WJ, Choi MS, Suh GY, Chung MP, Kim H, Kwon OJ. Lee BH, et al. Chest. 2005 Apr;127(4):1304-11. doi: 10.1378/chest.127.4.1304. Chest. 2005. PMID: 15821209 - Drug-induced hepatotoxicity of anti-tuberculosis drugs and their serum levels.
Jeong I, Park JS, Cho YJ, Yoon HI, Song J, Lee CT, Lee JH. Jeong I, et al. J Korean Med Sci. 2015 Feb;30(2):167-72. doi: 10.3346/jkms.2015.30.2.167. Epub 2015 Jan 21. J Korean Med Sci. 2015. PMID: 25653488 Free PMC article. - Antituberculosis drug-induced hepatotoxicity: concise up-to-date review.
Tostmann A, Boeree MJ, Aarnoutse RE, de Lange WC, van der Ven AJ, Dekhuijzen R. Tostmann A, et al. J Gastroenterol Hepatol. 2008 Feb;23(2):192-202. doi: 10.1111/j.1440-1746.2007.05207.x. Epub 2007 Nov 6. J Gastroenterol Hepatol. 2008. PMID: 17995946 Review. - Hepatotoxicity of antitubercular treatments. Rationale for monitoring liver status.
Durand F, Jebrak G, Pessayre D, Fournier M, Bernuau J. Durand F, et al. Drug Saf. 1996 Dec;15(6):394-405. doi: 10.2165/00002018-199615060-00004. Drug Saf. 1996. PMID: 8968694 Review.
Cited by
- Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications.
Sharifi-Rad J, Rayess YE, Rizk AA, Sadaka C, Zgheib R, Zam W, Sestito S, Rapposelli S, Neffe-Skocińska K, Zielińska D, Salehi B, Setzer WN, Dosoky NS, Taheri Y, El Beyrouthy M, Martorell M, Ostrander EA, Suleria HAR, Cho WC, Maroyi A, Martins N. Sharifi-Rad J, et al. Front Pharmacol. 2020 Sep 15;11:01021. doi: 10.3389/fphar.2020.01021. eCollection 2020. Front Pharmacol. 2020. PMID: 33041781 Free PMC article. Review. - Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) - validation of the Ayurvedic pharmacology through experimental and clinical studies.
Upadhyay AK, Kumar K, Kumar A, Mishra HS. Upadhyay AK, et al. Int J Ayurveda Res. 2010 Apr;1(2):112-21. doi: 10.4103/0974-7788.64405. Int J Ayurveda Res. 2010. PMID: 20814526 Free PMC article. - Tinospora cordifolia (Giloy): An insight on the multifarious pharmacological paradigms of a most promising medicinal ayurvedic herb.
Gupta A, Gupta P, Bajpai G. Gupta A, et al. Heliyon. 2024 Feb 15;10(4):e26125. doi: 10.1016/j.heliyon.2024.e26125. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38390130 Free PMC article. Review. - Do Tinospora cordifolia Cause Hepatic Damage?
Ruknuddin G, Narayanam S, Nesari TM. Ruknuddin G, et al. J Clin Exp Hepatol. 2022 Jan-Feb;12(1):244. doi: 10.1016/j.jceh.2021.09.006. Epub 2022 Jan 12. J Clin Exp Hepatol. 2022. PMID: 35068810 Free PMC article. No abstract available. - On the health effects of curcumin and its derivatives.
Ayub H, Islam M, Saeed M, Ahmad H, Al-Asmari F, Ramadan MF, Alissa M, Arif MA, Rana MUJ, Subtain M, Rahim MA, Zongo E, Ahmad N. Ayub H, et al. Food Sci Nutr. 2024 Sep 24;12(11):8623-8650. doi: 10.1002/fsn3.4469. eCollection 2024 Nov. Food Sci Nutr. 2024. PMID: 39620006 Free PMC article. Review.
References
- Runyon EH. Preventive Treatment in Tuberculosis: A Statement by the Committee on Therapy. American Thoracic Society. Am Rev Respir Dis. 1965;91:297–298. - PubMed
- Garibaldi RA, Drusin RE, Ferebee SH, Gregg MB. Isoniazid-associated hepatitis. Report of an outbreak. Am Rev Respir Dis. 1972;106:357–365. - PubMed
- Kopanoff DE, Snider DE Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. - PubMed
- Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical