Pbx/Meis deficiencies demonstrate multigenetic origins of congenital heart disease - PubMed (original) (raw)
Pbx/Meis deficiencies demonstrate multigenetic origins of congenital heart disease
Kryn Stankunas et al. Circ Res. 2008.
Abstract
Congenital heart diseases are traditionally considered to be multifactorial in pathogenesis resulting from environmental and genetic interactions that determine penetrance and expressivity within a genetically predisposed family. Recent evidence suggests that genetic contributions have been significantly underestimated. However, single gene defects occur only in a minority of cases, and multigenetic causes of congenital heart diseases have not been fully demonstrated. Here, we show that interactions between alleles of 3 Pbx genes, which encode homeodomain transcription factors, are sufficient to determine the phenotypic presentation of congenital heart diseases in mice. A major role is served by Pbx1, whose inactivation results in persistent truncus arteriosus. Reduction or absence of Pbx2 or Pbx3 leads to Pbx1 haploinsufficiency and specific malformations that resemble tetralogy of Fallot, overriding aorta with ventricular septal defect, and bicuspid aortic valves. Disruption of Meis1, which encodes a Pbx DNA-binding partner, results in cardiac anomalies that resemble those caused by Pbx mutations. Each of the observed cardiac defects represents developmental abnormalities affecting distinct stages of cardiac outflow tract development and corresponds to specific types of human congenital heart disease. Thus, varied deficiencies in the Pbx gene family produce a full spectrum of cardiac defects involving the outflow tract, providing a framework for determining multigenetic causes of congenital heart anomalies.
Figures
Figure 1
Pbx proteins are widely present in tissues relevant to heart development. A through C, Immunohistochemical analysis of Pbx proteins at E9.5. Pbx1b (A) is present in neural crest cells (arrow), mesenchymal cells of the branchial arch region (asterisk), endocardial cells (arrowhead), and myocardial cells (double arrows) of the cardiac OFT. Pbx2 (B) and Pbx3 (C) display similar expression patterns at E9.5. D through F, Presence of Pbx proteins in the cardiac OFT at E12.5. Pbx1b (D) is present in endocardial (arrowhead) and mesenchymal cells (asterisk) of the endocardial cushions of the OFT. Pbx1b is downregulated in myocardial cells (double arrow) at E12.5. Pbx1b is present in the endothelial and vascular smooth muscle cells of the aorta (Ao) and main pulmonary artery (MPA). Pbx2 (E) is present in endocardial (arrowhead), myocardial (double arrow), and mesenchymal cells (asterisk) of the OFT. Pbx2 is also present in endothelial and vascular smooth muscle cells of the aorta and main pulmonary artery. Pbx3a (F) is present in endocardial (arrowhead) and mesenchymal cells (asterisk) of the cushion but downregulated in myocardial cells (double arrow). Pbx3a is present in endothelial but not vascular smooth muscle cells of the great arteries.
Figure 2
_Pbx1_−/− embryos display PTA. A and B, Transverse sections show a normal aorta and main pulmonary artery in a wild-type embryo (A) vs a common arterial trunk in a _Pbx1_−/− embryo (B). Ao indicates aorta; MPA, main pulmonary artery. C and D, Vascular casting of the great arteries emerging from the embryonic heart in wild-type (C) and _Pbx1_−/− (D) embryos at E14.5. LPA indicates left pulmonary artery. E through G, Serial histological sections show origins of the coronary and main pulmonary arteries in _Pbx1_−/− embryos at E14.5. RCA indicates right coronary artery; LCA, left coronary artery. H through J, Serial histological sections show the 3 truncal valve leaflets and cusps in _Pbx1_−/− embryos at E14.5. RAC indicates right anterior cusp; LAC, left anterior cusp; PC, posterior cusp. K, Schematic representation of the origins of coronary and pulmonary arteries and the truncal valve leaflets and cusps in _Pbx1_−/− embryos.
Figure 3
Pbx2 makes subsidiary contributions to the alignment of cardiac OFTs. A, Right ventricular angiography of a newborn Pbx1+/−_2_−/− mouse. Asterisk indicates a direct connection between the right ventricle (RV) and the ascending aorta (Asc. Ao). B, Exposure of the right ventricular cavity in A reveals a high ventricular septal defect (VSD) and an overriding aorta (OA). C and D, Serial sections through the subaortic region in newborn Pbx1+/−_2_−/− mice demonstrate an aorta that overrides both ventricles. Section plane in C is caudal to the plane in D. LVOT indicates left ventricular OFT.
Figure 4
Pbx1+/−_2_−/− mice have bicuspid semilunar valves. A and B, Casting of the aortic valves in newborn wild-type (A) and Pbx1+/−_2_−/− (B) mice. Asc. Ao indicates ascending aorta; LCA, left coronary artery; RCA, right coronary artery; LCC, left coronary cusp; RCC, right coronary cusp; NCC, noncoronary cusp. C and D, Histological sections show the tricuspid aortic valve in a wild-type (C) and bicuspid aortic valve in a Pbx1+/−_2_−/− embryo (D) at E14.5. RV indicates right ventricle. E and F, Histological sections show a tricuspid pulmonic valve in a newborn wild-type (E) mouse vs a bicuspid aortic valve in a newborn Pbx1+/−_2_−/− mouse (F). MPA indicates main pulmonary artery. G, Schematic representation of the trileaflet aortic and pulmonic valves. AV indicates aortic valve; PV, pulmonic valve; AC, anterior cusp; LPC, left posterior cusp; RPC, right posterior cusp. H, Schematic representation of the bicuspid aortic and pulmonic valves in Pbx1+/−_2_−/− mice. The noncoronary cusp (NCC) of the aortic valve and the anterior cusp (AC) of the pulmonic valve fail to develop.
Figure 5
Both Pbx2 and Pbx3 contribute to OFT septation and semilunar valve formation. A though C, Gross appearance of Pbx2_−/−_3+/− (A), Pbx1+/−2_−/− (B), and Pbx1+/−_2_−/−_3+/− (C) embryos at E14.5. Asterisk indicates generalized edema. D through F, Transverse sections of the RVOT at E14.5. Pbx2_−/−_3+/− (D) and Pbx1+/−2_−/− (E) embryos display patent RVOTs with slender pulmonic valve (PV) leaflets. The RVOT of Pbx1+/−_2_−/−_3+/− embryos (F) is narrowed and associated with malformed pulmonic valve leaflets. G though I, Transverse sections of the right (RV) and left (LV) ventricles at E14.5. Both Pbx2_−/−_3+/− (G) and Pbx1+/−2_−/− (H) mice have normal right and left ventricular walls. Pbx1+/−_2_−/− mice (H) exhibit a ventricular septal defect (asterisk). Pbx1+/−_2_−/−_3+/− embryos (i) have both a ventricular septal defect (asterisk) and right ventricular hypertrophy. J through L, Transverse sections of the aortic valve at E14.5. Pbx2_−/−_3+/− mice (J) have a normal trileaflet aortic valve. Pbx1+/−2_−/− mice (K) have a bicuspid aortic valve. Pbx1+/−_2_−/−_3+/− embryos (L) have malformed aortic valves (AV). NCC indicates noncoronary cusp; RCC, right coronary cusp; LCC, left coronary cusp.
Figure 6
_Meis1_-null embryos display an overriding aorta and ventricular septal defect. A through D, Hematoxylin/eosin-stained sections from littermate E14.5 wild-type and _Meis1_−/− embryos. The aorta connects to the left ventricular OFT (LVOT) (A) but not the RVOT (C) in a wild-type embryo. In _Meis1_−/− embryos, the aorta connects to both the left ventricular OFT (B) and RVOT (D). AV indicates aortic valve; VSD, ventricular septal defect.
Figure 7
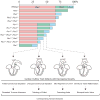
A semiquantitative model for the interactive roles of Pbx proteins in cardiac OFT morphogenesis. Pbx1 serves a major role in dictating conotruncal and semilunar valve development. Pbx2 and Pbx3 function to refine cardiac outflow morphogenesis. The bar chart indicates the proposed functional relative weight of Pbx proteins correlated with Pbx genotypes in cardiac development. Below, the cardiac phenotypes associated with specific Pbx genotypes are schematically depicted in decreasing order of severity, left to right. At the bottom, the human diseases that correspond to each developmental stage defined by Pbx deficiencies are listed.
Similar articles
- Meis1 and pKnox1 bind DNA cooperatively with Pbx1 utilizing an interaction surface disrupted in oncoprotein E2a-Pbx1.
Knoepfler PS, Calvo KR, Chen H, Antonarakis SE, Kamps MP. Knoepfler PS, et al. Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14553-8. doi: 10.1073/pnas.94.26.14553. Proc Natl Acad Sci U S A. 1997. PMID: 9405651 Free PMC article. - Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins.
Chang CP, Jacobs Y, Nakamura T, Jenkins NA, Copeland NG, Cleary ML. Chang CP, et al. Mol Cell Biol. 1997 Oct;17(10):5679-87. doi: 10.1128/MCB.17.10.5679. Mol Cell Biol. 1997. PMID: 9315626 Free PMC article. - A conserved motif N-terminal to the DNA-binding domains of myogenic bHLH transcription factors mediates cooperative DNA binding with pbx-Meis1/Prep1.
Knoepfler PS, Bergstrom DA, Uetsuki T, Dac-Korytko I, Sun YH, Wright WE, Tapscott SJ, Kamps MP. Knoepfler PS, et al. Nucleic Acids Res. 1999 Sep 15;27(18):3752-61. doi: 10.1093/nar/27.18.3752. Nucleic Acids Res. 1999. PMID: 10471746 Free PMC article. - Hox cofactors in vertebrate development.
Moens CB, Selleri L. Moens CB, et al. Dev Biol. 2006 Mar 15;291(2):193-206. doi: 10.1016/j.ydbio.2005.10.032. Epub 2006 Mar 3. Dev Biol. 2006. PMID: 16515781 Review. - PbX marks the spot.
Sagerström CG. Sagerström CG. Dev Cell. 2004 Jun;6(6):737-8. doi: 10.1016/j.devcel.2004.05.015. Dev Cell. 2004. PMID: 15177017 Review.
Cited by
- Brg1 governs distinct pathways to direct multiple aspects of mammalian neural crest cell development.
Li W, Xiong Y, Shang C, Twu KY, Hang CT, Yang J, Han P, Lin CY, Lin CJ, Tsai FC, Stankunas K, Meyer T, Bernstein D, Pan M, Chang CP. Li W, et al. Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1738-43. doi: 10.1073/pnas.1218072110. Epub 2013 Jan 14. Proc Natl Acad Sci U S A. 2013. PMID: 23319608 Free PMC article. - Zebrafish hoxd4a acts upstream of meis1.1 to direct vasculogenesis, angiogenesis and hematopoiesis.
Amali AA, Sie L, Winkler C, Featherstone M. Amali AA, et al. PLoS One. 2013;8(3):e58857. doi: 10.1371/journal.pone.0058857. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554940 Free PMC article. - Genome-Wide Analysis Identifies an Essential Human TBX3 Pacemaker Enhancer.
van Eif VWW, Protze SI, Bosada FM, Yuan X, Sinha T, van Duijvenboden K, Ernault AC, Mohan RA, Wakker V, de Gier-de Vries C, Hooijkaas IB, Wilson MD, Verkerk AO, Bakkers J, Boukens BJ, Black BL, Scott IC, Christoffels VM. van Eif VWW, et al. Circ Res. 2020 Dec 4;127(12):1522-1535. doi: 10.1161/CIRCRESAHA.120.317054. Epub 2020 Oct 12. Circ Res. 2020. PMID: 33040635 Free PMC article. - LncRNA-uc.40 silence promotes P19 embryonic cells differentiation to cardiomyocyte via the PBX1 gene.
Wu R, Xue P, Wan Y, Wang S, Gu M. Wu R, et al. In Vitro Cell Dev Biol Anim. 2018 Sep;54(8):600-609. doi: 10.1007/s11626-018-0284-0. Epub 2018 Aug 15. In Vitro Cell Dev Biol Anim. 2018. PMID: 30112697 - Reactivation of fetal splicing programs in diabetic hearts is mediated by protein kinase C signaling.
Verma SK, Deshmukh V, Liu P, Nutter CA, Espejo R, Hung ML, Wang GS, Yeo GW, Kuyumcu-Martinez MN. Verma SK, et al. J Biol Chem. 2013 Dec 6;288(49):35372-86. doi: 10.1074/jbc.M113.507426. Epub 2013 Oct 22. J Biol Chem. 2013. PMID: 24151077 Free PMC article.
References
- Pierpont ME, Basson CT, Benson DW, Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation. 2007;115:3015–3038. - PubMed
- Sandler T. Langman’s Medical Embryology. 10. Baltimore: Lippincott, Williams & Wilkins; 2006.
- Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med. 2000;342:334–342. - PubMed
- Kirby ML. Cardiac Development. 2007
- Rudolph AM. Congenital Diseases of the Heart: Clinical-Physiological Considerations. 2. New York: Futura Publishing Company; 2001.
Publication types
MeSH terms
Substances
Grants and funding
- R37 CA042971/CA/NCI NIH HHS/United States
- CA90735/CA/NCI NIH HHS/United States
- R01 CA090735/CA/NCI NIH HHS/United States
- CA42971/CA/NCI NIH HHS/United States
- R01 HL085345/HL/NHLBI NIH HHS/United States
- HL085345/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases