Dicer-dependent microRNA pathway safeguards regulatory T cell function - PubMed (original) (raw)
Dicer-dependent microRNA pathway safeguards regulatory T cell function
Adrian Liston et al. J Exp Med. 2008.
Abstract
Regulatory T (T reg) cells are indispensable for preventing autoimmunity. Incumbent to this role is the ability of T reg cells to exert their suppressor function under inflammatory conditions. We found that T reg cell-mediated tolerance is critically dependent on the Dicer-controlled microRNA (miRNA) pathway. Depletion of miRNA within the T reg cell lineage resulted in fatal autoimmunity indistinguishable from that in T reg cell-deficient mice. In disease-free mice lacking Dicer in all T cells or harboring both Dicer-deficient and -sufficient T reg cells, Dicer-deficient T reg cells were suppressive, albeit to a lesser degree, whereas their homeostatic potential was diminished as compared with their Dicer-sufficient counterparts. However, in diseased mice, Dicer-deficient T reg cells completely lost suppressor capacity. Thus, miRNA preserve the T reg cell functional program under inflammatory conditions.
Figures
Figure 1.
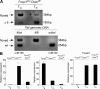
Deletion of a conditional Dicer allele and the resulting miRNA paucity in Foxp3+ CD4+ T cells from Foxp3CreDicerfl/fl mice. (A) An efficient Cre-mediated excision of the Dicerfl/fl allele in CD4+Foxp3+ cells, but not in CD4+Foxp3− cells, in Foxp3CreDicerfl/fl mice. The excised “floxed” allele produced a predominant 185-bp PCR product, whereas CD4+Foxp3− cells retained the intact floxed allele producing a 384-bp PCR product. In non–T reg (TR) cells (CD4+Foxp3−), we observed a weak signal corresponding to a deleted Dicerfl allele, in agreement with our recent finding that Cre-mediated recombination of a single floxed allele might occur in 1–5% of Foxp3− T cells in Foxp3Cre mice, whereas the deletion of both floxed alleles is likely negligible (reference 25). (B) Real-time PCR analysis of miR155, miR150, and Foxp3 mRNA expression in CD4+Foxp3−CD62Lhi TN cells and CD4+YFP+Foxp3+ T reg (TR) cells purified from Foxp3CreDicerfl/wt and Foxp3Cre/wtDicerfl/fl mice. The data are shown as the mean and standard deviation representing three independent experiments. miR155 was selected as an example of an miRNA with an increased expression in Foxp3+ cells compared with Foxp3−CD4+ T cells, and miR150 was selected as an example of an miRNA with a reduced expression in Foxp3+ cells.
Figure 2.
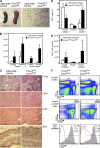
Fatal early onset autoimmunity in mice with T reg cell–specific Dicer deficiency. (A and B) Lymphadenopathy and splenomegaly in Foxp3CreDicerfl/fl mice. A 4-fold increase in spleen (P < 0.05) and a 6-fold increase in lymph node (P < 0.0005) cellularity in 3-wk-old mutant mice (n = 8 per group) occurred; a 3-fold increase in spleen (P < 0.005) and a 14-fold increase in lymph node (P < 0.0001) cellularity in 4-wk-old mutant mice (n = 3–15 per group) occurred compared with the littermate controls. Bar, 1 cm. (C) Histopathology of lung, liver, and skin in Foxp3CreDicerfl/fl mice. Hematoxylin and eosin–stained tissue sections of 3-wk-old Foxp3CreDicerfl/fl mice showed massive lymphocytic infiltrates and disrupted tissue architecture compared with Foxp3CreDicerwt/fl littermates. Sections shown are representative of three individual mice per group. Bars, 100 μm. (D) The proportion of naive Foxp3−CD62Lhi (TN), Foxp3+ T reg, and effector Foxp3−CD62Llow (TE) cells within the lymph node CD4+ T cell population in Foxp3CreDicerfl/fl mice and littermate controls. (E) Absolute numbers of CD62Lhi TN, CD62LlowTE, and Foxp3+ T reg lymph node cells. (F) Analysis of lymph node T cell subsets in Foxp3CreDicerfl/fl and control Foxp3CreDicerwt/fl mice for CD4, CD8, Foxp3, CD62L, and CD25 expression (percentages of cells in the indicated gates are shown). CD25 histograms include TN (red line), TE (blue line), and T reg (shaded) populations (n = 11–18). Values shown are means ± standard deviation.
Figure 3.
Augmented cytokine production and elevated serum levels of IgE in mice harboring Dicer-deficient T reg cells. (A and B) Flow cytometric analysis of IL-2 and IFN-γ production by splenic T cells in Foxp3creDicerfl/fl and control mice induced upon 5 h of in vitro stimulation with 1 μg/ml CD3 and 1 μg/ml CD28 antibodies. The results are representative of three independent experiments with two to four mice per group (percentages of cells in the indicated gates are shown). (C) Quantification of serum Ig isotype levels in Dicerwt/wt (n = 6), Foxp3KO (n = 4), CD4-Cre Dicerfl/fl (n = 4), and Foxp3CreDicerfl/fl (n = 5) mice using ELISA. Foxp3CreDicerfl/fl and Foxp3KO mice exhibited ∼10-fold elevation in serum IgM and IgG1 and even higher elevation in serum IgE. ND, not detected. In these experiments, the control group included serum samples from both B6 mice as a control for Foxp3ko and from Dicer-sufficient littermate control mice. The two control groups did not show significant differences in Ig isotype levels in two independent experiments (B6, n = 5; Dicer littermate controls, n = 13). Values shown are means ± standard deviation.
Figure 4.
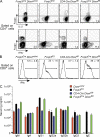
Reduced homeostatic potential of Dicer-deficient T reg cells. In female Foxp3Cre/wt heterozygous mice, random X chromosome inactivation results in two distinct subsets of T reg cells: a YFP− population expressing the wild-type Foxp3 protein and lacking in Cre activity, and a YFP+ population expressing Foxp3Cre. (A and B) Comparable size of thymic and peripheral YFP+Foxp3+ T reg cell subsets, as well as peripheral CD62LhiFoxp3− TN, Foxp3+ T reg, and CD62LlowFoxp3− TE subsets in Foxp3Cre/wtDicerfl/fl and Foxp3Cre/wtDicerfl/wt mice (n = 7–8 per group). Values shown are means ± standard deviation. (C) Flow cytometric analysis of CD4, CD8, and Foxp3 expression by CD4 single-positive thymocytes, and CD4 and YFP expression by Foxp3+ single-positive thymocytes. (D) Flow cytometric analysis of CD62L and Foxp3 expression by peripheral CD4+ cells, and CD4 and YFP expression by peripheral Foxp3+ cells (n = 6 per group). (E) Dicer-deficient T reg cells (YFP+) from healthy Foxp3Cre/wtDicerfl/fl or control Foxp3Cre/wtDicerfl/wt mice were stained for Ki67 upon ex vivo isolation or after 24 h of in vitro stimulation in the presence of CD3 and CD28 antibodies and IL-2. (F) Apoptosis was measured by staining for active caspase-3 using FITC-VAD-FMK after in vitro stimulation for the indicated periods of time. Some activated cells were restimulated with anti-CD3 for an additional 24 h before the apoptosis analysis. Percentages of cells in the indicated gates are shown in C–F.
Figure 5.
A complete loss of suppressive capacity of Dicer-deficient T reg cells in diseased mice. (A) Dicer-deficient T reg (TR) cells (YFP+) isolated from healthy control Foxp3Cre/wtDicerfl/fl mice and autoimmune Foxp3CreDicerfl/fl mice, as well as wild-type Foxp3CreDicerwt/wt littermate controls, were co-cultured with responder CD4 T cells at the indicated ratios for 72 h in the presence of 1 μg/ml CD3 antibody and irradiated T cell–depleted splenocytes. (B) Dicer-deficient T reg cells (GFP+) isolated from CD4-Cre Dicerfl/fl mice were examined in comparison with T reg cells from sick Foxp3creDicerfl/fl mice and wild-type Foxp3CreDicerwt/wt littermate controls. Values shown are means ± standard deviation.
Figure 6.
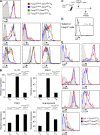
Altered phenotypic features and diminished expression of putative T reg cell suppressor molecules in the absence of the Dicer-dependent miRNA pathway. (A) Expression of Foxp3, CD25, IL-7Rα, GITR, CTLA4, ICOS, and CD103 on Dicer-deficient T reg (TR) cells, wild-type littermate YFP+ T reg cells (Foxp3Cre/wtDicerfllwt), YFP+ Foxp3Cre/wtDicerfl/fl T reg cells, and YFP+ Foxp3CreDicerfl/fl T reg cells compared with TN cells from wild-type littermates. (B) Expression of IL-10, Ebi3, TGF-β, and granzyme B mRNA in Dicer-deficient T reg (TR) cells (CD4+YFP+; Foxp3Cre/wtDicerfl/fl), wild-type littermate TN cells (CD4+CD25−CD62Lhi), and T reg cells (CD4+YFP+; Foxp3Cre/wtDicerfllwt). Values shown are means ± standard deviation. (C) Ly5.1+ T reg cells were transferred to Foxp3CreDicerfl/fl mice. 5 d after transfer, mice were killed and analyzed by flow cytometry. (D) Resident Dicer-deficient and transferred Dicer-sufficient T reg cells were gated by CD4, Foxp3, and Ly5.1 from total lymphocytes isolated from peripheral lymph nodes (the percentage of cells in the indicated gate is shown). (E) Expression of Ki67, CD25, CD62L, CD73, GITR, CTLA4, and ICOS on recipient Dicer-deficient T reg (TR) cells, transferred wild-type T reg cells (Ly5.1+Dicerwtlwt), and Foxp3− TE cells.
Similar articles
- Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity.
Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Zhou X, et al. J Exp Med. 2008 Sep 1;205(9):1983-91. doi: 10.1084/jem.20080707. Epub 2008 Aug 25. J Exp Med. 2008. PMID: 18725525 Free PMC article. - Dicer insufficiency and microRNA-155 overexpression in lupus regulatory T cells: an apparent paradox in the setting of an inflammatory milieu.
Divekar AA, Dubey S, Gangalum PR, Singh RR. Divekar AA, et al. J Immunol. 2011 Jan 15;186(2):924-30. doi: 10.4049/jimmunol.1002218. Epub 2010 Dec 13. J Immunol. 2011. PMID: 21149603 Free PMC article. - Dicer-dependent microRNAs control maturation, function, and maintenance of Langerhans cells in vivo.
Kuipers H, Schnorfeil FM, Fehling HJ, Bartels H, Brocker T. Kuipers H, et al. J Immunol. 2010 Jul 1;185(1):400-9. doi: 10.4049/jimmunol.0903912. Epub 2010 Jun 7. J Immunol. 2010. PMID: 20530258 - Dicing with viruses: microRNAs as antiviral factors.
Müller S, Imler JL. Müller S, et al. Immunity. 2007 Jul;27(1):1-3. doi: 10.1016/j.immuni.2007.07.003. Immunity. 2007. PMID: 17663977 Review. - Dicer cuts the kidney.
Ho JJ, Marsden PA. Ho JJ, et al. J Am Soc Nephrol. 2008 Nov;19(11):2043-6. doi: 10.1681/ASN.2008090986. Epub 2008 Oct 15. J Am Soc Nephrol. 2008. PMID: 18923053 Review. No abstract available.
Cited by
- Dynamic modulation of thymic microRNAs in response to stress.
Belkaya S, Silge RL, Hoover AR, Medeiros JJ, Eitson JL, Becker AM, de la Morena MT, Bassel-Duby RS, van Oers NS. Belkaya S, et al. PLoS One. 2011;6(11):e27580. doi: 10.1371/journal.pone.0027580. Epub 2011 Nov 16. PLoS One. 2011. PMID: 22110677 Free PMC article. - Dicing the Disease with Dicer: The Implications of Dicer Ribonuclease in Human Pathologies.
Theotoki EI, Pantazopoulou VI, Georgiou S, Kakoulidis P, Filippa V, Stravopodis DJ, Anastasiadou E. Theotoki EI, et al. Int J Mol Sci. 2020 Sep 30;21(19):7223. doi: 10.3390/ijms21197223. Int J Mol Sci. 2020. PMID: 33007856 Free PMC article. Review. - MicroRNA-31 negatively regulates peripherally derived regulatory T-cell generation by repressing retinoic acid-inducible protein 3.
Zhang L, Ke F, Liu Z, Bai J, Liu J, Yan S, Xu Z, Lou F, Wang H, Zhu H, Sun Y, Cai W, Gao Y, Li Q, Yu XZ, Qian Y, Hua Z, Deng J, Li QJ, Wang H. Zhang L, et al. Nat Commun. 2015 Jul 13;6:7639. doi: 10.1038/ncomms8639. Nat Commun. 2015. PMID: 26165721 Free PMC article. - Treg functional stability and its responsiveness to the microenvironment.
Barbi J, Pardoll D, Pan F. Barbi J, et al. Immunol Rev. 2014 May;259(1):115-39. doi: 10.1111/imr.12172. Immunol Rev. 2014. PMID: 24712463 Free PMC article. Review. - Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation.
Beyer M, Thabet Y, Müller RU, Sadlon T, Classen S, Lahl K, Basu S, Zhou X, Bailey-Bucktrout SL, Krebs W, Schönfeld EA, Böttcher J, Golovina T, Mayer CT, Hofmann A, Sommer D, Debey-Pascher S, Endl E, Limmer A, Hippen KL, Blazar BR, Balderas R, Quast T, Waha A, Mayer G, Famulok M, Knolle PA, Wickenhauser C, Kolanus W, Schermer B, Bluestone JA, Barry SC, Sparwasser T, Riley JL, Schultze JL. Beyer M, et al. Nat Immunol. 2011 Aug 14;12(9):898-907. doi: 10.1038/ni.2084. Nat Immunol. 2011. PMID: 21841785 Free PMC article.
References
- Hori, S., T. Nomura, and S. Sakaguchi. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061. - PubMed
- Khattri, R., T. Cox, S.A. Yasayko, and F. Ramsdell. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4:337–342. - PubMed
- Fontenot, J.D., M.A. Gavin, and A.Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336. - PubMed
- Fontenot, J.D., J.P. Rasmussen, L.M. Williams, J.L. Dooley, A.G. Farr, and A.Y. Rudensky. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341. - PubMed
- Brunkow, M.E., E.W. Jeffery, K.A. Hjerrild, B. Paeper, L.B. Clark, S.A. Yasayko, J.E. Wilkinson, D. Galas, S.F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases