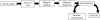Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells - PubMed (original) (raw)
Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells
Liying Yang et al. Biotechnol Bioeng. 2009.
Abstract
The optimization of a purely negative depletion, enrichment process for circulating tumor cells (CTCs) in the peripheral blood of head and neck cancer patients is presented. The enrichment process uses a red cell lysis step followed by immunomagnetic labeling, and subsequent depletion, of CD45 positive cells. A number of relevant variables are quantified, or attempted to be quantified, which control the performance of the enrichment process. Six different immunomagnetic labeling combinations were evaluated as well as the significant difference in performance with respect to the blood source: buffy coats purchased from the Red Cross, fresh, peripheral blood from normal donors, and fresh peripheral blood from human cancer patients. After optimization, the process is able to reduce the number of normal blood cells in a cancer patient's blood from 4.05 x 10(9) to 8.04 x 10(3) cells/mL and still recover, on average, 2.32 CTC per mL of blood. For all of the cancer patient blood samples tested in which CTC were detected (20 out of 26 patients) the average recovery of CTCs was 21.7 per mL of blood, with a range of 282 to 0.53 CTC. Since the initial number of CTC in a patient's blood is unknown, and most probably varies from patient to patient, the recovery of the CTC is unknown. However, spiking studies of a cancer cell line into normal blood, and subsequent enrichment using the optimized protocol indicated an average recovery of approximately 83%. Unlike a majority of other published studies, this study focused on quantifying as many factors as possible to facilitate both the optimization of the process as well as provide information for current and future performance comparisons. The authors are not aware any other reported study which has achieved the performance reported here (a 5.66 log(10)) in a purely negative enrichment mode of operation. Such a mode of operation of an enrichment process provides significant flexibility in that it has no bias with respect to what attributes define a CTC; thereby allowing the researcher or clinician to use any maker they choose to define whether the final, enrich product contains CTCs or other cell type relevant to the specific question (i.e., does the CTC have predominantly epithelial or mesenchymal characteristics?).
Figures
Figure 1
Flow diagram of current process to enrich for rare cancer cells in human blood.
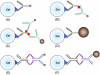
Figure 2
Six immunomagnetic labeling protocols used in this study. Specifics of protocols A through F are listed in Table 2.
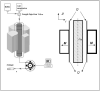
Figure 3
Diagram of the immunomagnetic cell separation system used in this study.
Figure 4
Photographs of microscopic images of a cytospin of one of the enriched peripheral blood samples from cancer patients. Figure 4A is a brightfield image, 4B is an image filtered for FTIC staining, 4C is a image filtered for DAPI staining, and 4D is an electronic superposition of images 4B and 4C. Original magnification ×200. 4E is a photo of two gels of RT-PCR product, the first lane is an example of positive presence of EGFR, the second lane an example of a negative presence of EDFR, and the third lane is molecular weight control.
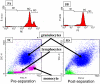
Figure 5
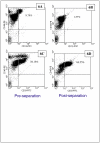
Flow cytometry analysis of one of the cancer patient blood samples, labeled with Protocol A, prior to (5A, 5C), and after (5B and 5D), immunomagnetic cell separation. 5A and 5B are histograms of PE expression of cells labeled with an anti-CD45-PE, and 5C and 5D are dot plots of forward scatter of the cell sample prior to and after separation. Based on the three gated regions, M1, M2, and M3, in 5A and 5B, the “dots” in 5C and 5D are color coded corresponding to M1 (green), M2 (blue), and M3 (magenta).
Figure 6
Flow cytometry analysis of one of the cancer patient blood samples, labeled with Protocol A, prior to (6A, 6C), and after (6B and 6D), immunomagnetic cell separation. In this example, two different sets of immunomagnetic labeling was conducted: 6A and 6B presents cells labeled with anti-CD45-PE and anti-CD13-FITC, and 6C and 6D presents cells labeled with anti-CD45-PE and anti-CD33-FITC.
Figure 7
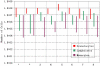
A bar graph representing the immunomagnetic depletion of the three general categories of WBC, granulocytes, lymphocytes, and monocytes, for eight different cancer patients blood samples. Labeling protocol A was used for all eight depletions.
Figure 8
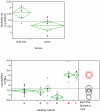
JMP software graphical presentation of immunomagnetic depletions as measured by the log10 depletion of nucleated cells. Figure 8A compares the depletion results of blood from buffy coats and cancer patients, both blood sample immunomagnetically labeled with Protocol A. 8B compares the depletion results (n = 61) of blood from cancer patients, buffy coat, and normal individuals using the six different labeling protocols presented in Figure 2.
Similar articles
- Application of immunomagnetic cell enrichment in combination with RT-PCR for the detection of rare circulating head and neck tumor cells in human peripheral blood.
Tong X, Yang L, Lang JC, Zborowski M, Chalmers JJ. Tong X, et al. Cytometry B Clin Cytom. 2007 Sep;72(5):310-23. doi: 10.1002/cyto.b.20177. Cytometry B Clin Cytom. 2007. PMID: 17205568 - Negative enrichment by immunomagnetic nanobeads for unbiased characterization of circulating tumor cells from peripheral blood of cancer patients.
Liu Z, Fusi A, Klopocki E, Schmittel A, Tinhofer I, Nonnenmacher A, Keilholz U. Liu Z, et al. J Transl Med. 2011 May 19;9:70. doi: 10.1186/1479-5876-9-70. J Transl Med. 2011. PMID: 21595914 Free PMC article. - Significance of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: initial results.
Jatana KR, Balasubramanian P, Lang JC, Yang L, Jatana CA, White E, Agrawal A, Ozer E, Schuller DE, Teknos TN, Chalmers JJ. Jatana KR, et al. Arch Otolaryngol Head Neck Surg. 2010 Dec;136(12):1274-9. doi: 10.1001/archoto.2010.223. Arch Otolaryngol Head Neck Surg. 2010. PMID: 21173379 Free PMC article. - Emerging technologies for CTC detection based on depletion of normal cells.
Lustberg M, Jatana KR, Zborowski M, Chalmers JJ. Lustberg M, et al. Recent Results Cancer Res. 2012;195:97-110. doi: 10.1007/978-3-642-28160-0_9. Recent Results Cancer Res. 2012. PMID: 22527498 Free PMC article. Review. - Immunomagnetic separation technologies.
Hoeppener AE, Swennenhuis JF, Terstappen LW. Hoeppener AE, et al. Recent Results Cancer Res. 2012;195:43-58. doi: 10.1007/978-3-642-28160-0_4. Recent Results Cancer Res. 2012. PMID: 22527493 Review.
Cited by
- Simultaneous, single particle, magnetization and size measurements of micron sized, magnetic particles.
Xu J, Mahajan K, Xue W, Winter JO, Zborowski M, Chalmers JJ. Xu J, et al. J Magn Magn Mater. 2012 Dec 1;324(24):4189-4199. doi: 10.1016/j.jmmm.2012.07.039. J Magn Magn Mater. 2012. PMID: 22962515 Free PMC article. - Assessment of γ-H2AX levels in circulating tumor cells from patients receiving chemotherapy.
Garcia-Villa A, Balasubramanian P, Miller BL, Lustberg MB, Ramaswamy B, Chalmers JJ. Garcia-Villa A, et al. Front Oncol. 2012 Oct 25;2:128. doi: 10.3389/fonc.2012.00128. eCollection 2012. Front Oncol. 2012. PMID: 23112954 Free PMC article. - Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments.
Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, El-Rifai W, Bedognetti D, Batra SK, Haris M, Bhat AA, Macha MA. Lone SN, et al. Mol Cancer. 2022 Mar 18;21(1):79. doi: 10.1186/s12943-022-01543-7. Mol Cancer. 2022. PMID: 35303879 Free PMC article. Review. - Technologies for circulating tumor cell separation from whole blood.
Bankó P, Lee SY, Nagygyörgy V, Zrínyi M, Chae CH, Cho DH, Telekes A. Bankó P, et al. J Hematol Oncol. 2019 May 14;12(1):48. doi: 10.1186/s13045-019-0735-4. J Hematol Oncol. 2019. PMID: 31088479 Free PMC article. Review. - Heterogeneous atypical cell populations are present in blood of metastatic breast cancer patients.
Lustberg MB, Balasubramanian P, Miller B, Garcia-Villa A, Deighan C, Wu Y, Carothers S, Berger M, Ramaswamy B, Macrae ER, Wesolowski R, Layman RM, Mrozek E, Pan X, Summers TA, Shapiro CL, Chalmers JJ. Lustberg MB, et al. Breast Cancer Res. 2014 Mar 6;16(2):R23. doi: 10.1186/bcr3622. Breast Cancer Res. 2014. PMID: 24602188 Free PMC article.
References
- Berx G, Raspe E, Christofori G, Thiery JP, Sleeman JP. Pre-EMTing metastasis ? Recapitulation of morphogenetic processes in cancer. Clin. Exp. Metastasis. 2007;24:587–597. - PubMed
- Brandt BH, Schmidt H, de Angelis G, Zänker Predictive laboratory diagnostics in oncology utilizing blood-borne cancer cells- current best practice and unmet needs. Cancer Lett. 2001;162:S11–S16. - PubMed
- Brazin R, Lemieux R, Tremblay T, St-Amour I. Tetramolecular immune complexes are more effectient than IVIg to prevent antibody-dependent in vitro and in vivo phagocytosis of blood cells. British J. of Haematology. 2004;127:90–96. - PubMed
- Braun S, Pantel K. Clinical significance of occult metastatic cells in bone marrow of breast cancer patients. Oncologist. 2001;6(2):125–132. - PubMed
- Chosy J, Melnik K, Comella K, Zborowski M, Chalmers JJ. Characterization of Antibody Binding to Three Cancer-Related Antigens Using Flow Cytometry and Cell Tracking Velocimetry. Biotechnol Bioeng. 2003;82:340–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA062349/CA/NCI NIH HHS/United States
- R01 CA97391-01A1/CA/NCI NIH HHS/United States
- R01 CA62349/CA/NCI NIH HHS/United States
- R33 CA81662-01/CA/NCI NIH HHS/United States
- R33 CA081662/CA/NCI NIH HHS/United States
- R01 CA097391/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous