Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling - PubMed (original) (raw)
Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling
Joshua J Breunig et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Primary cilia are present on mammalian neurons and glia, but their function is largely unknown. We generated conditional homozygous mutant mice for a gene we termed Stumpy. Mutants lack cilia and have conspicuous abnormalities in postnatally developing brain regions, including a hypoplasic hippocampus characterized by a primary deficiency in neural stem cells known as astrocyte-like neural precursors (ALNPs). Previous studies suggested that primary cilia mediate sonic hedgehog (Shh) signaling. Here, we find that loss of ALNP cilia leads to abrogated Shh activity, increased cell cycle exit, and morphological abnormalities in ALNPs. Processing of Gli3, a mediator of Shh signaling, is also altered in the absence of cilia. Further, key mediators of the Shh pathway localize to ALNP cilia. Thus, selective targeting of Shh machinery to primary cilia confers to ALNPs the ability to differentially respond to Shh mitogenic signals compared to neighboring cells. Our data suggest these organelles are cellular "antennae" critically required to modulate ALNP behavior.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
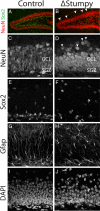
Gross hippocampal defects in Stumpy_-deficient brain. (A and B) Immunostaining at P13 for NeuN revealed an overall smaller granule cell layer (GCL) and dispersed neurons (white arrowheads) in Stumpy_-mutant (Δ_Stumpy) compared to control brains. (C and D) NeuN-labeled neuronal nuclei showed reduced thickness of the GCL in mutant (D) compared to heterozygous littermates (C). White arrowheads denote dispersed granule cells. (E and F) Sox2-labeled nuclei were dispersed in Δ_Stumpy (F) compared to control (E). (G and H) GFAP immunostaining in Δ_Stumpy_ brain (H) revealed a dramatic reduction of radial glia and fiber density compared to control (G). Remaining GFAP+ cells in Δ_Stumpy_ mice had abnormal morphology characterized by disorganized and misoriented processes. (I and J) DAPI-nuclear staining showed decreased cell density in both the SGZ and GCL of mutants (J) compared to control (I).
Fig. 2.
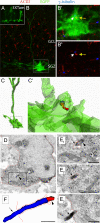
Cilia are present on hippocampal ALNPs. (A) GFAP-CRE-EGFP transgenic mice were injected with tamoxifen for three days and sacrifed one day after the last injection. An example of mosaic labeling of glia with EGFP is shown. (B) EGFP-expressing radial glial cell (boxed) in the subgranular zone (SGZ) in conjunction with immunstaining for ACIII (red) and γ-tubulin (blue). Cilia were located in both GCL and SGZ. (B′) Higher magnification of the inset in B. A cilium was observed extending from the EGFP+ cell expressing both γ-tubulin (white arrow) and ACIII (yellow arrow). (B″) The inset in B showing γ-tubulin (white arrow) and ACIII (yellow arrow). Note that the cilia length is much shorter than overlying GCL cells. (C, C′) Z-stack data were used to reconstruct the cell in B and the location of the cilum within the cell membrane (C′). The spatial resolution of this technique is limited by the technology. Nevertheless, the cilium appears to be largely enveloped in the EGFP+ membrane. (D) An astrocyte glial cell harboring a cilium in the SGZ of a P7 wild-type mouse. Low power electron micrograph of a GFAP+ cell detected with diffuse anti-GFAP DAB immunoprecipitation in the cytoplasm. The framed area is depicted in serial high power micrographs in E. (E) Examples of the basal body (arrowhead) and the ACIII+ axoneme (arrow) detected with black Ni-intensified DAB-immunoprecipitation. (F) Three-dimensional-reconstruction of the cilium showing the basal body (red; arrowhead) and the axoneme (blue; arrow). The cilium was followed in 12 consecutive serial sections until its termination. The total length of the axoneme (∼1.7 μm) was measured in 3D reconstruction. The cell membrane is approximately outlined by green dotted lines. Scale bars, 0.5 μm.
Fig. 3.
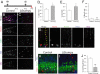
Altered cell cycle dynamics, proliferation and differentiation. (A) Control or Δ_Stumpy_ mice were injected with CldU at P8, IdU on P12 and killed at P13. (B and C) Immunostaining for Mcm2 (red), Ki67 (magenta), Idu (blue), and Cldu (green) in control showed predominant labeling within the SGZ and the GCL. Conversely, Δ_Stumpy_ mice exhibited diffusely distributed proliferating cells and an overall reduction of proliferating cells. Arrows in B point to the Mcm2+ population that does not colocalize with the other proliferative markers, indicative of slowly dividing cells in G1 of the cell cycle. Note the complete lack of this population in C. (D) The percentage of cells that were CldU+ and Idu− was higher in Δ_Stumpy_ brains, indicating that less of the proliferating pool at P8 is in the cell cycle at P12. (E) The percentage of cells that were Idu+ and Ki67− at P13. In Δ_Stumpy_ mice, the proportion of cells that exited the cell cycle after 24 h is increased compared with controls. (F) The percentage of cells that were Mcm2+ and negative for Ki67, CldU, and IdU at P13. This slowly dividing stem/precursor pool is largely depleted in Δ_Stumpy_ mice. (G) Nestin+/Gfap+/Sox2+ cells—radial and nonradial glia—make up a large population of the Mcm2 population in controls (white arrowheads). Note the attached Nestin+/Gfap+ radial process (yellow arrowheads). (H–J) The reduction of dividing cells [labeled at P8 with CldU (red)] results in a reduced number of DCX (blue) and NeuN- (green) positive neurons (white arrowheads) in Δ_Stumpy_ mutant DG (I) compared to control (H)—quantified in (J). Non-neuronal CldU+ cells are pointed out by yellow arrowheads. *P < 0.05; **P < 0.001 (Student's t test).
Fig. 4.
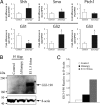
Altered mRNA levels of Shh pathway genes and changes in Gli3 processing. (A) Expression levels of Shh pathway genes in hippocampus using qRT-PCR. Levels of Shh, Smo, Ptc, and Gli1–3 were normalized to levels of HPRT. *P < 0.05 (Student's t test). (B) Western blot showing the levels of full-length Gli3 (∼190 kDa) in P5 hippocampus (Hipp) of control and Δ_Stumpy_ compared to E11.5 fetus. Note the absence of a detectable band in the control lane. Shown below is the β-actin loading control. (C) The blot in (B) was repeated in triplicate and the graph shows the average intensity of Gli3-190 signal relative to β-actin signal for the indicated groups.
Fig. 5.
Localization of Shh molecules to ALNP cilia. (A and B) Immunostaining for Gli1, Sox2 and GFAP in control (A) and Δ_Stumpy_ (B) mutants. Gli1 largely colocalized with both Sox2 and GFAP in control. Gli1 immunostaining was reduced in Δ_Stumpy_ brains. White arrowheads denote Gli1 cytoplasm/nuclei. Yellow arrowheads denote cytoplasmic Gli1. Red arrowheads denote presumptive ciliary Gli1. Purple arrowhead in B and B′ denotes a single, faint Gli1+ nucleus in a Sox2− cell. Gli1 immunostaining channel from A and B is shown from control (A′) and Δ_Stumpy_ (B′) tissue. (C and D) Higher magnification of boxed region in (A). Shown in (C) is a single optical section from the orthographic view shown in (D). The Gli1 enrichment (white arrowhead) resembles localization to a cilium. (E, E′) Immunostaining of Smo (green), γ-tubulin (red), Sox2 (blue), and Gfap (magenta) in a pair of hilar astrocytes near the SGZ. Smo appears enriched in short cilia charateristic of ALNPs. (E′) is a higher magnification of boxed region in E.
Similar articles
- Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain.
Gazea M, Tasouri E, Tolve M, Bosch V, Kabanova A, Gojak C, Kurtulmus B, Novikov O, Spatz J, Pereira G, Hübner W, Brodski C, Tucker KL, Blaess S. Gazea M, et al. Dev Biol. 2016 Jan 1;409(1):55-71. doi: 10.1016/j.ydbio.2015.10.033. Epub 2015 Nov 2. Dev Biol. 2016. PMID: 26542012 Free PMC article. - Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components.
Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT. Qin J, et al. Proc Natl Acad Sci U S A. 2011 Jan 25;108(4):1456-61. doi: 10.1073/pnas.1011410108. Epub 2011 Jan 5. Proc Natl Acad Sci U S A. 2011. PMID: 21209331 Free PMC article. - Cilia-dependent GLI processing in neural crest cells is required for tongue development.
Millington G, Elliott KH, Chang YT, Chang CF, Dlugosz A, Brugmann SA. Millington G, et al. Dev Biol. 2017 Apr 15;424(2):124-137. doi: 10.1016/j.ydbio.2017.02.021. Epub 2017 Mar 9. Dev Biol. 2017. PMID: 28286175 Free PMC article. - The relationship between sonic Hedgehog signaling, cilia, and neural tube defects.
Murdoch JN, Copp AJ. Murdoch JN, et al. Birth Defects Res A Clin Mol Teratol. 2010 Aug;88(8):633-52. doi: 10.1002/bdra.20686. Birth Defects Res A Clin Mol Teratol. 2010. PMID: 20544799 Free PMC article. Review. - Cilia, ciliopathies and hedgehog-related forebrain developmental disorders.
Andreu-Cervera A, Catala M, Schneider-Maunoury S. Andreu-Cervera A, et al. Neurobiol Dis. 2021 Mar;150:105236. doi: 10.1016/j.nbd.2020.105236. Epub 2020 Dec 28. Neurobiol Dis. 2021. PMID: 33383187 Review.
Cited by
- Characterization of primary cilia during the differentiation of retinal ganglion cells in the zebrafish.
Lepanto P, Davison C, Casanova G, Badano JL, Zolessi FR. Lepanto P, et al. Neural Dev. 2016 Apr 6;11:10. doi: 10.1186/s13064-016-0064-z. Neural Dev. 2016. PMID: 27053191 Free PMC article. - Depletion of primary cilia from mature dentate granule cells impairs hippocampus-dependent contextual memory.
Rhee S, Kirschen GW, Gu Y, Ge S. Rhee S, et al. Sci Rep. 2016 Sep 28;6:34370. doi: 10.1038/srep34370. Sci Rep. 2016. PMID: 27678193 Free PMC article. - Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation.
Higginbotham H, Guo J, Yokota Y, Umberger NL, Su CY, Li J, Verma N, Hirt J, Ghukasyan V, Caspary T, Anton ES. Higginbotham H, et al. Nat Neurosci. 2013 Aug;16(8):1000-7. doi: 10.1038/nn.3451. Epub 2013 Jun 30. Nat Neurosci. 2013. PMID: 23817546 Free PMC article. - Contribution of Smoothened Receptor Signaling in GABAergic Neurotransmission and Chloride Homeostasis in the Developing Rodent Brain.
Hamze M, Medina I, Delmotte Q, Porcher C. Hamze M, et al. Front Physiol. 2021 Dec 10;12:798066. doi: 10.3389/fphys.2021.798066. eCollection 2021. Front Physiol. 2021. PMID: 34955901 Free PMC article. Review. - Stress-induced localization of HSPA6 (HSP70B') and HSPA1A (HSP70-1) proteins to centrioles in human neuronal cells.
Khalouei S, Chow AM, Brown IR. Khalouei S, et al. Cell Stress Chaperones. 2014 May;19(3):321-7. doi: 10.1007/s12192-013-0459-2. Epub 2013 Sep 6. Cell Stress Chaperones. 2014. PMID: 24061851 Free PMC article.
References
- Sawamoto K, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–632. - PubMed
- Berbari NF, Bishop GA, Askwith CC, Lewis JS, Mykytyn K. Hippocampal neurons possess primary cilia in culture. J Neurosci Res. 2007;85:1095–1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 AG029726/AG/NIA NIH HHS/United States
- R00 AG029726/AG/NIA NIH HHS/United States
- 4R00AG029726-02/AG/NIA NIH HHS/United States
- 1K99AG029726-01/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases