Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage - PubMed (original) (raw)
Review
Mass spectrometric quantification of amino acid oxidation products identifies oxidative mechanisms of diabetic end-organ damage
Anuradha Vivekanadan-Giri et al. Rev Endocr Metab Disord. 2008 Dec.
Abstract
Diabetes mellitus is increasingly prevalent worldwide. Diabetic individuals are at markedly increased risk for premature death due to cardiovascular disease. Furthermore, substantial morbidity results from microvascular complications which include retinopathy, nephropathy, and neuropathy. Clinical studies involving diabetic patients have suggested that degree of diabetic hyperglycemia correlates with risk of complications. Recent evidence implicates a central role for oxidative stress and vascular inflammation in all forms of insulin resistance, obesity, diabetes and its complications. Although, glucose promotes glycoxidation reactions in vitro and products of glycoxidation and lipoxidation are elevated in plasma and tissue in diabetics, the exact relationships among hyperglycemia, the diabetic state, and oxidative stress are not well-understood. Using a combination of in vitro and in vivo experiments, we have identified amino acid oxidation markers that serve as molecular fingerprints of specific oxidative pathways. Quantification of these products utilizing highly sensitive and specific gas chromatography/mass spectrometry in animal models of diabetic complications and in humans has provided insights in oxidative pathways that result in diabetic complications. Our studies strongly support the hypothesis that unique oxidants are generated in the microenvironment of tissues vulnerable to diabetic damage. Potential therapies interrupting these reactive pathways in target tissue are likely to be beneficial in preventing diabetic complications.
Figures
Fig. 1
Oxidative stress pathways in diabetic complications. AGE advanced glycosylation end-products, ALE advanced lipoxidation end-products, eNOS endothelial nitric oxide synthase, HOCl hypochlorous acid, MPO myeloperoxidase, _NO_• nitric oxide, NOX NAD(P)H oxidase, PUFA polyunsaturated fatty acid, XO xanthine oxidase. Modified from [26]
Fig. 2
Quantification of _ortho_-tyrosine (a), _meta_-tyrosine (b), _o,o′_-dityrosine (c), and 3-nitrotyrosine (d) in aortic proteins isolated from control and diabetic Cynomolgus monkeys. Aortic tissue was harvested from control and diabetic animals at the end of the 6-month study. Tissue was delipidated, hydrolyzed, and amino acids were quantified by GC/MS. *p<0.01 by Student's _t_-test. Reproduced from [29]
Fig. 3
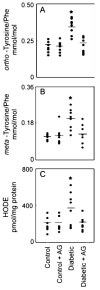
Quantification of oxidized amino acids and lipids in retinal tissue isolated from control and hyperglycemic Sprague–Dawley rats. Rats were rendered hyperglycemic with STZ. At the end of the 6-week study, retinal tissue was harvested from the animals. The isolated amino acids were derivatized and analyzed by GC/MS with selected ion monitoring (a and b). HODEs (hydroxyoctadecadienoic acid) were quantified by reversed-phase HPLC (c). *p<0.05 by analysis of variance. Reproduced from [28]
Fig. 4
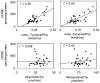
Correlation of _ortho_-tyrosine, _meta_-tyrosine _o,o′_-dityrosine, or 3-nitrotyrosine with HODEs in retinal tissue of control and diabetic Sprague–Dawley rats. At the end of the 6-week study, retinal tissue harvested from control animals, control animals treated with aminoguanidine, diabetic animals, and diabetic animals treated with aminoguanidine was analyzed for oxidation products. Lines represent the linear least-squares fit of the data. Reproduced from [28]
Fig. 5
MPO generates chlorinated and nitrated HDL in human plasma in subjects with CAD. Levels of MPO-oxidized amino acids were determined in HDL isolated from healthy subjects and subjects with CAD. a 3-chlorotyrosine and b 3-nitrotyrosine. Linear regression analysis demonstrated a strong correlation between levels of 3-chlorotyrosine and levels of 3-nitrotyrosine (c) in plasma HDL consistent with similar pathway of generation of both these markers. Reproduced from [50, 60]
Similar articles
- Mechanisms for oxidative stress in diabetic cardiovascular disease.
Pennathur S, Heinecke JW. Pennathur S, et al. Antioxid Redox Signal. 2007 Jul;9(7):955-69. doi: 10.1089/ars.2007.1595. Antioxid Redox Signal. 2007. PMID: 17508917 Review. - Glycation as the glucose link to diabetic complications.
Gugliucci A. Gugliucci A. J Am Osteopath Assoc. 2000 Oct;100(10):621-34. J Am Osteopath Assoc. 2000. PMID: 11105451 Review. - Mechanisms of oxidative stress in diabetes: implications for the pathogenesis of vascular disease and antioxidant therapy.
Pennathur S, Heinecke JW. Pennathur S, et al. Front Biosci. 2004 Jan 1;9:565-74. doi: 10.2741/1257. Front Biosci. 2004. PMID: 14766391 Review. - Oxidized amino acids: culprits in human atherosclerosis and indicators of oxidative stress.
Heinecke JW. Heinecke JW. Free Radic Biol Med. 2002 Jun 1;32(11):1090-101. doi: 10.1016/s0891-5849(02)00792-x. Free Radic Biol Med. 2002. PMID: 12031894 Review. - The oxidative stress in the development of diabetes chronic complications in the elderly.
Suciu I, Negrean V, Sâmpelean D. Suciu I, et al. Rom J Intern Med. 2004;42(2):395-406. Rom J Intern Med. 2004. PMID: 15529629
Cited by
- Spinach Methanolic Extract Attenuates the Retinal Degeneration in Diabetic Rats.
Bautista-Pérez R, Cano-Martínez A, Gutiérrez-Velázquez E, Martínez-Rosas M, Pérez-Gutiérrez RM, Jiménez-Gómez F, Flores-Estrada J. Bautista-Pérez R, et al. Antioxidants (Basel). 2021 May 3;10(5):717. doi: 10.3390/antiox10050717. Antioxidants (Basel). 2021. PMID: 34063668 Free PMC article. - Decreased nitric oxide bioavailability in a mouse model of Fabry disease.
Shu L, Park JL, Byun J, Pennathur S, Kollmeyer J, Shayman JA. Shu L, et al. J Am Soc Nephrol. 2009 Sep;20(9):1975-85. doi: 10.1681/ASN.2008111190. Epub 2009 Jul 23. J Am Soc Nephrol. 2009. PMID: 19628671 Free PMC article. - Kinetic studies on the reaction between dicyanocobinamide and hypochlorous acid.
Maitra D, Ali I, Abdulridha RM, Shaeib F, Khan SN, Saed GM, Pennathur S, Abu-Soud HM. Maitra D, et al. PLoS One. 2014 Nov 6;9(11):e110595. doi: 10.1371/journal.pone.0110595. eCollection 2014. PLoS One. 2014. PMID: 25375773 Free PMC article. - Association of plasma ortho-tyrosine/para-tyrosine ratio with responsiveness of erythropoiesis-stimulating agent in dialyzed patients.
Kun S, Mikolás E, Molnár GA, Sélley E, Laczy B, Csiky B, Kovács T, Wittmann I. Kun S, et al. Redox Rep. 2014 Sep;19(5):190-8. doi: 10.1179/1351000214Y.0000000090. Epub 2014 Apr 3. Redox Rep. 2014. PMID: 24693974 Free PMC article. - Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid.
Pennathur S, Maitra D, Byun J, Sliskovic I, Abdulhamid I, Saed GM, Diamond MP, Abu-Soud HM. Pennathur S, et al. Free Radic Biol Med. 2010 Jul 15;49(2):205-13. doi: 10.1016/j.freeradbiomed.2010.04.003. Epub 2010 Apr 11. Free Radic Biol Med. 2010. PMID: 20388538 Free PMC article.
References
- American Diabetes Association. Diabetes Statistics. http://www.diabetes.org/diabetes-statistics.jsp.
- Centers for Disease Control and Prevention. US Department of Health and Human Services. Centers for Disease Control and Prevention; Atlanta GA: 2004.
Publication types
MeSH terms
Substances
Grants and funding
- P50 DK039255/DK/NIDDK NIH HHS/United States
- R21 HL092237/HL/NHLBI NIH HHS/United States
- P30 AG024824/AG/NIA NIH HHS/United States
- R21HL092237/HL/NHLBI NIH HHS/United States
- P50DK039255/DK/NIDDK NIH HHS/United States
- P30AG024824/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical