Short telomeres are a risk factor for idiopathic pulmonary fibrosis - PubMed (original) (raw)
. 2008 Sep 2;105(35):13051-6.
doi: 10.1073/pnas.0804280105. Epub 2008 Aug 27.
Julian J-L Chen, Lisa Lancaster, Sonye Danoff, Shu-chih Su, Joy D Cogan, Irma Vulto, Mingyi Xie, Xiaodong Qi, Rubin M Tuder, John A Phillips 3rd, Peter M Lansdorp, James E Loyd, Mary Y Armanios
Affiliations
- PMID: 18753630
- PMCID: PMC2529100
- DOI: 10.1073/pnas.0804280105
Short telomeres are a risk factor for idiopathic pulmonary fibrosis
Jonathan K Alder et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Idiopathic interstitial pneumonias (IIPs) have a progressive and often fatal course, and their enigmatic etiology has complicated approaches to effective therapies. Idiopathic pulmonary fibrosis (IPF) is the most common of IIPs and shares with IIPs an increased incidence with age and unexplained scarring in the lung. Short telomeres limit tissue renewal capacity in the lung and germ-line mutations in telomerase components, hTERT and hTR, underlie inheritance in a subset of families with IPF. To examine the hypothesis that short telomeres contribute to disease risk in sporadic IIPs, we recruited patients who have no family history and examined telomere length in leukocytes and in alveolar cells. To screen for mutations, we sequenced hTERT and hTR. We also reviewed the cases for features of a telomere syndrome. IIP patients had shorter leukocyte telomeres than age-matched controls (P < 0.0001). In a subset (10%), IIP patients had telomere lengths below the first percentile for their age. Similar to familial cases with mutations, IPF patients had short telomeres in alveolar epithelial cells (P < 0.0001). Although telomerase mutations were rare, detected in 1 of 100 patients, we identified a cluster of individuals (3%) with IPF and cryptogenic liver cirrhosis, another feature of a telomere syndrome. Short telomeres are thus a signature in IIPs and likely play a role in their age-related onset. The clustering of cryptogenic liver cirrhosis with IPF suggests that the telomere shortening we identify has consequences and can contribute to what appears clinically as idiopathic progressive organ failure in the lung and the liver.
Conflict of interest statement
Conflict of interest statement: P.M.L. is a founding shareholder in Repeat Diagnostics, a company that specializes in length measurement of leukocyte telomeres with the use of flow FISH.
Figures
Fig. 1.
Telomere length in lymphocytes from IIP patients and families with known telomerase mutations compared with healthy controls. (A) IIP patients, in yellow, have shorter telomeres than age-matched controls (P < 0.0001, Wilcoxon signed rank). IIP patients [60 of 62 (97%)] have telomeres shorter than the median of healthy controls (P < 0.0001). Of 62 IIP patients, 50 (81%) carried the diagnosis of IPF. (B) Detailed view of A with individuals with features of a telomere syndrome highlighted in red: *, a 77-year-old IPF patient with hTR 325G→T mutation; §, a patient with very short telomeres who had chronic unexplained thrombocytopenia, a feature of subclinical aplastic anemia; ‡, two individuals with both IPF and cryptogenic liver cirrhosis who have short telomeres. Ten percent of IIP patients (6 of 62) have short telomeres below the first percentile; a range predictive of the presence of a telomerase mutation. (C) Telomere length from 45 individuals from 10 families with known mutations in hTERT (n = 17), hTR (n = 3), and DKC1 (n = 4). (D) Bar graph illustrates the mean difference in telomere length from the median of age-matched healthy controls. Compared with noncarriers whose telomere length was similar to controls (P = 0.304, Wilcoxon signed rank), both sporadic IIP patients and known telomerase mutation carriers had shorter telomeres (P < 0.0001 for both).
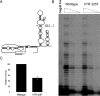
Fig. 2.
Germ-line hTR mutation in an IPF patient with no family history. (A) Secondary structure of hTR. hTR 325 G→T predicts disrupting the integrity of the conserved P5 helix and is thus expected to compromise function. (B) Gel of in vitro reconstituted telomerase with 5-fold dilutions as indicated. Telomerase activity of mutant hTR is compromised as shown by the decreased intensity of the repeat ladder compared with wild type. Quantitation of three independent experiments shown in C indicates that this allele is hypomorphic. Hypomorphic alleles of hTERT and hTR have been previously described in both aplastic anemia and familial IPF patients (5, 6, 18).
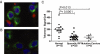
Fig. 3.
Telomere length quantitation in alveolar epithelium by FISH. Lung cells from patients with usual interstitial pneumonia have short telomeres. (A) Representative images of nuclei of surfactant positive C cells (cytoplasmic staining in green) from an individual with no known lung disease showing bright telomere signals after hybridizing with a fluorescent telomere probe (pink). In contrast, alveolar cells from a patient with IPF have significantly shorter telomeres as seen by the dim or absent telomere signal in B. (C) Telomere signal per alveolar cell from age-matched individuals with normal lungs, sporadic IPF, and known hTERT (n = 4) or hTR mutation (n = 1) carriers. Mean age per group was 61, 59, and 64 years, respectively. IPF patients, with and without a family history, have shorter telomeres than controls with P values, as shown.
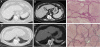
Fig. 4.
Imaging and pathology from patients with both idiopathic pulmonary fibrosis and cryptogenic liver cirrhosis. Computed tomography A and D shows honeycomb changes of IPF in the lung bases. B and E show representative abnormalities in the same patients with evidence of decompensated cirrhosis with splenomegaly and portal hypertension in B and nodular and abnormal liver contour in E associated with intraoperative description of a cirrhotic liver. (C and F) Reticulin stains of liver explants from a patient with scans in A and B and of a second patient who underwent liver transplant 3 years before his IPF diagnosis. The fibrosis on the background of cirrhotic lobules is prominent in the interstitial and perivascular space.
References
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
- Loyd JE. Pulmonary fibrosis in families. Am J Respir cell Mol Biol. 2003;29:S47–S50. - PubMed
- Thomas AQ, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. - PubMed
- Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical