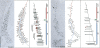TOR regulates the subcellular distribution of DIM2, a KH domain protein required for cotranscriptional ribosome assembly and pre-40S ribosome export - PubMed (original) (raw)
TOR regulates the subcellular distribution of DIM2, a KH domain protein required for cotranscriptional ribosome assembly and pre-40S ribosome export
Emmanuel Vanrobays et al. RNA. 2008 Oct.
Abstract
Eukaryotic ribosome synthesis is a highly dynamic process that involves the transient association of scores of trans-acting factors to nascent pre-ribosomes. Many ribosome synthesis factors are nucleocytoplasmic shuttling proteins that engage the assembly pathway at early nucleolar stages and escort pre-ribosomes to the nucleoplasm and/or the cytoplasm. Here, we report that two 40S ribosome synthesis factors, the KH-domain protein DIM2 and the HEAT-repeats/Armadillo-domain and export factor RRP12, are nucleolar restricted upon nutritional, osmotic, and oxidative stress. Nucleolar entrapment of DIM2 and RRP12 was triggered by rapamycin treatment and was under the strict control of the target of rapamycin (TOR) signaling cascade. DIM2 binds pre-rRNAs directly through its KH domain at the 5'-end of ITS1 (D-A(2) segment) and, consistent with its requirements in early nucleolar pre-rRNA processing, is required for efficient cotranscriptional ribosome assembly. The substitution of a single and highly conserved amino acid (G207A) within the KH motif is sufficient to inhibit pre-rRNA processing in a fashion similar to genetic depletion of DIM2. DIM2 carries an evolutionarily conserved putative nuclear export sequence (NES) at its carboxyl-terminal end that is required for efficient pre-40S ribosome export. Strikingly, DIM2 and RRP12 are both involved in the nucleocytoplasmic translocation of pre-ribosomes, suggesting that this step in the ribosome assembly pathway has been selected as a regulatory target for the TOR pathway.
Figures
FIGURE 1.
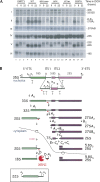
The KH domain of DIM2 adopts the canonical β1-α1-α2-β2-β3-α3 fold and is essential for protein function. (A) The KH domain of DIM2 is evolutionarily conserved. Sc, Saccharomyces cerevisiae; Ap, Aeropyrum pernix; Sp, Schizosaccharomyces pombe; Dm, Drosophila melanogaster; Hs, Homo sapiens. The KH domain extends from residue 179 to 252. The most conserved region (referred to here as “core domain”) extends from residue 203 to 213; consensus (GXXIGXXGXXI; where X is any amino acid) shown at the bottom. The various regions of the KH domain are arbitrarily color-coded and annotated (closed and open diamonds and triangles) according to our functional mapping analysis (see below). Regions predicted to adopt helices (H), strands (E), and coils (C) are annotated. Residues conserved in KH domains are shaded in pink; identical or highly similar residues are highlighted in gray. (B) The three-dimensional structure of the KH domain of DIM2 was homology modeled based on the 1.5-Å resolution crystal structure of an archaeal homolog from Aeropyrum pernix (Jia et al. 2007; see Materials and Methods). Color code and annotations are as in panel A; central residues of the core subdomain are highlighted by their three-letter code. (C) Functional mapping of the KH domain. Each DIM2 construct was expressed from a low-copy (ARS/CEN-LEU2) plasmid as an amino-tagged Protein A fusion. The ΔKH core mutation carries an 11 amino acid deletion (residues 203–213). The ΔC ter mutation carries a premature stop codon at position 203. In the ΔKH allele, the whole KH domain encompassing residues 179–252 is deleted. The G207A mutation harbors a single amino acid substitution. (D) Steady-state levels of DIM2 constructs and effects of DIM2 mutations on growth. Each DIM2 mutation was tested for its effects on growth in liquid (top panel) and solid (middle panel) cultures in tet∷dim2 cells upon the addition of doxycycline. X axis, time of transfer to doxycycline (up to 48 h); Y axis, OD600/OD600 at 0 min expressed in a log scale. The doubling time is indicated for the wild-type strain and the G207A substitution. SD, dextrose synthetic medium lacking leucine; +DOX, SD supplemented with 10 μg/mL of doxycycline. (Bottom panel) Each DIM2 construct was stably expressed. Equivalent amounts of total protein extracted in the absence of doxycycline-induced depletion were loaded in each lane and tested by anti-ProtA Western blot analysis.
FIGURE 2.
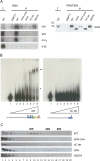
The KH domain of DIM2 is required for pre-rRNA processing at sites A1 and A2. (A) Pre-rRNA processing analysis. A single alanine substitution in the KH domain of DIM2 at the conserved position 207 (G207A) was sufficient to mimic the inhibitory effects on pre-rRNA processing of protein depletion. Steady-state levels of pre-rRNA and mature rRNA in tet∷dim2 cells transformed with the DIM2 constructs depicted in Figure 1C, as well as with an empty control plasmid, are shown. Total RNA was extracted from cells growing exponentially in selective medium (SD −leu) in the absence (0-h time points) or following the addition of doxycycline (8 and 22 h). Total RNA was separated on denaturing agarose gels and transferred to nylon membranes for Northern blotting. Oligonucleotides used in the hybridization are described in Supplemental Table 3 and in panel B. Panels I and V were hybridized with probe b, panel II with probe c, panels III with probe e, panel IV with probe f, and panel VI with probe a. (B) Primary Pol I transcript (35S) and simplified pre-rRNA processing pathway in S. cerevisiae. The mature 18S, 5.8S, and 25S rRNA are interspersed with external transcribed spacers (5′-ETS and 3′-ETS), as well as with internal transcribed spacers (ITS1 and ITS2). Cleavage sites (A0 to E) are indicated, as are probes a to f used in the Northern blot hybridizations and primer extension analysis. The position of the probe used in the FISH analysis at the 5′ end of ITS1 is represented in red. Three initial nucleolar cleavages at sites A0, A1, and A2 generate successively the 33S, the 32S, and the 20S and 27SA2 pre-rRNAs. The 20S pre-rRNA is exported to the cytoplasm, where it is dimethylated by DIM1 and cleaved at site D, generating mature 18S rRNA. The 27SA2 pre-rRNA undergoes two alternative processing pathways, only one of which—the major, accounting for up to 80% of total pre-rRNA processing—is represented here. Cleavage at site A3 by RNAse MRP is followed by 5′–3′ exoribonucleolytic digestion by RAT1 and XRN1 to B1S. Endonucleolytic cleavage at site C2 in ITS2 is followed by C2 to E digestion by the Exosome and C2 to C1 digestion by RAT1 and XRN1. A full description can be found in Lafontaine (2004) and Henras et al. (2008). (Inset) 22S RNA.
FIGURE 3.
The KH domain of DIM2 binds the D-A2 region of the pre-rRNA in vivo and in vitro. (A) Affinity purification analysis. Total extracts from yeast cells expressing the DIM2 constructs described in Figure 1 were subjected to affinity purification with IgG-coupled agarose beads. Copurifying RNAs were identified either by primer extension or Northern blotting (left panels). As a control, the presence of each bait protein in the pellet fractions was tested by Western blotting (right panel). (Left panels) RNA aliquots of the total (T) and pellet (P) fractions, loaded in a 1:10 ratio, were either processed by primer extension (33S detected with oligo a) or by Northern blots with separation in a 1.2% agarose/formaldehyde gel (20S) or in an 8% acrylamide/urea gel (D-A2 and 5.8S). Gels were transferred to nylon membranes and hybridized in a Northern blot experiment. The 20S pre-rRNA and the D-A2 spacer fragment were detected with probe b (Fig. 2B). As a control for specificity, a probe that selects the 5.8S rRNA (oligonucleotide d) that is not known to interact with DIM2 was used. (Right panel) Protein aliquots of the total (T) and pellet (P) fractions, loaded in a 1:10 ratio, were separated by SDS-PAGE and transferred for Western blotting with an antibody specific to the protein A moiety of the protein fusions. (B) Gel-shift assay. Increasing amounts (0–250 nM) of purified recombinant full-length GST-DIM2 and a version lacking the carboxyl-terminal KH domain were incubated with 10 fmol of gel-purified, radiolabeled, in vitro synthesized D-A2 fragment and resolved on 5% acrylamide gels in nondenaturing conditions (see Materials and Methods). Shift (*) and supershift (**) are indicated. (C) Glycerol gradient analysis. Equivalent amounts of total extracts prepared from cells expressing the DIM2 constructs indicated were layered onto 10%–30% glycerol gradients and resolved by centrifugation. Twenty fractions were recovered, and total protein was extracted and analyzed by anti-ProtA Western blot.
FIGURE 4.
DIM2 is required for cotranscriptional ribosome assembly. Representative yeast rRNA genes are shown as visualized by chromatin spreading. tet∷dim2 cells were grown to mid-log phase in YPD (control) or YPD supplemented with doxycycline (17-h deplete) and spreads made according to Osheim et al. (2004). Interpretive tracing of the genes and transcript mapping are provided. DNA is color coded as follows: 5′ end to site A2 in red, A2 to 3′ end in blue, and the intergene spacer in green. Particles that appear on the transcripts are shown on the tracing as follows: gray particles correspond to the initial small 5′-terminal knobs, red to the mature SSU processomes, and blue to pre-large-subunit knobs that form at the 5′ end of cleaved transcripts. Scale bar is 0.5 μm.
FIGURE 5.
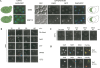
DIM2 and RRP12 are nucleolar-restricted upon nutritional, osmotic, and oxidative stress. (A) GFP fluorescence in fixed cells. Yeast cells expressing functional GFP constructs of DIM2 and RRP12 grown in complete glucose-based medium were observed by fluorescence in fast-growing conditions and in saturated cultures. Cells were collected at OD600 0.3 (EXPO) and 2 d following the diauxic-shift (SATURATION). Both DIM2 and RRP12 showed a nucleolar staining facing the bulk DNA labeled with DAPI. As expected, mitochondrial DNA (cytoplasmic ring) was detected in cells from saturated cultures (best seen in the RRP12 panel). DIC, differential interference contrast; DAPI, DNA stain; GFP, green fluorescence. Summary cartoons to the left and right. No, nucleolus; Np, nucleoplasm. (B,C) GFP fluorescence in live cells. Hypertonic and oxidative stresses mimic the effect of nutrient deprivation on DIM2 and RRP12 nucleolar relocation. Fast-growing DIM2-GFP or RRP12-GFP cells were exposed to various stresses, including NaCl 0.5 M, DTT 2 mM, DMSO 10%, and H2O2 0.4 mM, and directly observed by GFP fluorescence at regular intervals. For each stress, a time course is illustrated. The crescent-shaped nucleolar labeling is easily scored. (D) Indirect immunofluorescence. Nucleolar sequestration was triggered by treating fast-growing yeast cells expressing DIM2-GFP or RRP12-GFP with NaCl for 30 min. Spheroplasts were decorated with an anti-Nop1 antibody and counterstained with DAPI.
FIGURE 6.
The nucleolar entrapment of DIM2 and RRP12 is under TOR control. (A) GFP fluorescence in live cells. DIM2-GFP cells expressing either a wild-type (TOR1) or a semi-dominant (tor1-1) allele of TOR1 or carrying a deletion in FPR1 (fpr1∷Δ) were cultured to mid log-phase (OD600 ∼0.3), exposed to 200 mM rapamycin for up to 2 h and directly observed by GFP fluorescence at the time points indicated (right panels). Both fpr1∷Δ and tor1-1 cells were resistant to rapamycin in a drop plate assay (data not shown). As a control, all strains were grown to saturation (2 d post-diauxic shift) in the absence of rapamycin treatment (left panels). The crescent-shaped staining indicative of nucleolar localization was easily scored. EXPO, fast growing yeast cells; SAT, saturated cultures. For the time points for each condition, the upper photos are differential interference contrast and the lower photos are fluorescence micrographs. (B) Indirect immunofluorescence. DIM2-GFP and RRP12-GFP cells were either grown exponentially (OD600 ∼0.3), grown exponentially and exposed to rapamycin treatment for 60 min, or grown to saturation. They were then processed in an immunofluorescence experiment with an antibody specific to the fibrillarin (mA66 against yeast Nop1, see Materials and Methods), which was decorated in red. (C) GFP fluorescence in live cells. In the absence of a functional KH domain the subcellular distribution and trafficking of DIM2-GFP is affected. Diploid cells expressing either a full-length carboxyl GFP version of DIM2 (WT) or a version carrying a truncation of the KH domain (ΔC ter) were cultured in complete medium and imaged by fluorescence in fast-growing yeast cells (OD600 ∼0.3, EXPO) and in saturated cultures (2 d post-diauxic shift, SAT) (left panels). In parallel, exponentially growing cells were exposed to a final concentration of 200 mM rapamycin for the time points indicated (right panels).
FIGURE 7.
DIM2 contains a putative NES sequence and is required for pre-40S ribosome export. (A) DIM2 carries an evolutionarily conserved putative leucine-rich NES. Sc, Saccharomyces cerevisiae; Dm, Drosophila melanogaster; Hs, Homo sapiens. (B) Pre-40S ribosomes visualization by FISH in mutants in the putative NES. Depletion of endogenous DIM2 was achieved by transferring tet∷dim2 cells transformed with a wild-type full-length construct (WT), an empty control plasmid, or constructs expressing either a precise deletion of the putative NES (ΔNES) or versions with alanine substitutions in conserved residues (L177A and L177AL180A) in doxycycline-containing medium for 12 and 24 h. All panels shown were hybridized with a Cy3-labeled 5′-ITS1 probe (oligo LD597). Arrowheads point to the cytoplasm; closed arrowhead points to nucleoplasm. (Inset) The 12 h depletion time point is shown with differential interference contrast (DIC), DNA stain (DAPI), and overlay. (C) Detailed functional characterization of the ΔNES mutation. The expression of a DIM2 construct that precisely lacks the six amino acid residues that encompass the putative NES was sufficient to severely inhibit pre-40S export in conditions that did not significantly affect pre-rRNA processing. (Left panels) Pre-rRNAs were visualized as in panel B. Depletion of endogenous DIM2 was achieved for 1 h. Arrowhead points to the cytoplasm. (Right top panels) The ΔNES construct is stably expressed. Equivalent amount of total protein extracted in the absence of doxycycline-induced depletion was loaded in each lane and tested by anti-ProtA Western blot analysis. As a loading control, the membrane was probed with an antibody specific to G-6-PDH. (Right bottom panels) Depleting endogenous DIM2 for 1 h did not result in any significant pre-rRNA processing inhibitions at sites A1–A2. Pre-rRNA processing analysis was conducted as described in Figure 2.
Similar articles
- Interdependent action of KH domain proteins Krr1 and Dim2 drive the 40S platform assembly.
Sturm M, Cheng J, Baßler J, Beckmann R, Hurt E. Sturm M, et al. Nat Commun. 2017 Dec 20;8(1):2213. doi: 10.1038/s41467-017-02199-4. Nat Commun. 2017. PMID: 29263326 Free PMC article. - Rrp12 and the Exportin Crm1 participate in late assembly events in the nucleolus during 40S ribosomal subunit biogenesis.
Moriggi G, Nieto B, Dosil M. Moriggi G, et al. PLoS Genet. 2014 Dec 4;10(12):e1004836. doi: 10.1371/journal.pgen.1004836. eCollection 2014 Dec. PLoS Genet. 2014. PMID: 25474739 Free PMC article. - TOR regulates late steps of ribosome maturation in the nucleoplasm via Nog1 in response to nutrients.
Honma Y, Kitamura A, Shioda R, Maruyama H, Ozaki K, Oda Y, Mini T, Jenö P, Maki Y, Yonezawa K, Hurt E, Ueno M, Uritani M, Hall MN, Ushimaru T. Honma Y, et al. EMBO J. 2006 Aug 23;25(16):3832-42. doi: 10.1038/sj.emboj.7601262. Epub 2006 Aug 3. EMBO J. 2006. PMID: 16888624 Free PMC article. - Assembly of the small ribosomal subunit in yeast: mechanism and regulation.
Chaker-Margot M. Chaker-Margot M. RNA. 2018 Jul;24(7):881-891. doi: 10.1261/rna.066985.118. Epub 2018 Apr 30. RNA. 2018. PMID: 29712726 Free PMC article. Review. - Inside the 40S ribosome assembly machinery.
Karbstein K. Karbstein K. Curr Opin Chem Biol. 2011 Oct;15(5):657-63. doi: 10.1016/j.cbpa.2011.07.023. Epub 2011 Aug 20. Curr Opin Chem Biol. 2011. PMID: 21862385 Free PMC article. Review.
Cited by
- RNA Export through the NPC in Eukaryotes.
Okamura M, Inose H, Masuda S. Okamura M, et al. Genes (Basel). 2015 Mar 20;6(1):124-49. doi: 10.3390/genes6010124. Genes (Basel). 2015. PMID: 25802992 Free PMC article. Review. - Ribosome biogenesis factors-from names to functions.
Dörner K, Ruggeri C, Zemp I, Kutay U. Dörner K, et al. EMBO J. 2023 Apr 3;42(7):e112699. doi: 10.15252/embj.2022112699. Epub 2023 Feb 10. EMBO J. 2023. PMID: 36762427 Free PMC article. Review. - Interdependent action of KH domain proteins Krr1 and Dim2 drive the 40S platform assembly.
Sturm M, Cheng J, Baßler J, Beckmann R, Hurt E. Sturm M, et al. Nat Commun. 2017 Dec 20;8(1):2213. doi: 10.1038/s41467-017-02199-4. Nat Commun. 2017. PMID: 29263326 Free PMC article. - Hierarchical recruitment into nascent ribosomes of assembly factors required for 27SB pre-rRNA processing in Saccharomyces cerevisiae.
Talkish J, Zhang J, Jakovljevic J, Horsey EW, Woolford JL Jr. Talkish J, et al. Nucleic Acids Res. 2012 Sep 1;40(17):8646-61. doi: 10.1093/nar/gks609. Epub 2012 Jun 26. Nucleic Acids Res. 2012. PMID: 22735702 Free PMC article. - Human PDCD2L Is an Export Substrate of CRM1 That Associates with 40S Ribosomal Subunit Precursors.
Landry-Voyer AM, Bilodeau S, Bergeron D, Dionne KL, Port SA, Rouleau C, Boisvert FM, Kehlenbach RH, Bachand F. Landry-Voyer AM, et al. Mol Cell Biol. 2016 Nov 28;36(24):3019-3032. doi: 10.1128/MCB.00303-16. Print 2016 Dec 15. Mol Cell Biol. 2016. PMID: 27697862 Free PMC article.
References
- Bradatsch, B., Katahira, J., Kowalinski, E., Bange, G., Yao, W., Sekimoto, T., Baumgartel, V., Boese, G., Bassler, J., Wild, K., et al. Arx1 functions as an unorthodox nuclear export receptor for the 60S preribosomal subunit. Mol. Cell. 2007;27:767–779. - PubMed
- Fatica, A., Tollervey, D. Making ribosomes. Curr. Opin. Cell Biol. 2002;14:313–318. - PubMed
- Fromont-Racine, M., Senger, B., Saveanu, C., Fasiolo, F. Ribosome assembly in eukaryotes. Gene. 2003;313:17–42. - PubMed
- Gadal, O., Strauss, D., Kessl, J., Trumpower, B., Tollervey, D., Hurt, E. Nuclear export of 60s ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p. Mol. Cell. Biol. 2001;21:3405–3415. - PMC - PubMed
- Garcia-Mena, J., Das, A., Sanchez-Trujillo, A., Portier, C., Montanez, C. A novel mutation in the KH domain of polynucleotide phosphorylase affects autoregulation and mRNA decay in Escherichia coli . Mol. Microbiol. 1999;33:235–248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases