Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma - PubMed (original) (raw)
Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma
Valentina Fogal et al. Cancer Res. 2008.
Abstract
A tumor homing peptide, LyP-1, selectively binds to tumor-associated lymphatic vessels and tumor cells in certain tumors and exhibits an antitumor effect. Here, we show that the protein known as p32 or gC1q receptor is the receptor for LyP-1. Various human tumor cell lines were positive for p32 expression in culture, and the expression was increased in xenograft tumors grown from the positive cell lines. Fluorescence-activated cell sorting analyses with anti-p32 antibodies showed that p32-positive cell lines expressed p32 at the cell surface. These cells bound and internalized LyP-1 peptide in proportion to the cell-surface expression level, which correlated with malignancy rather than total p32 expression in the cells. Like the LyP-1 peptide, p32 antibodies highlighted hypoxic areas in tumors, where they bound to both tumor cells and cells that expressed macrophage/myeloid cell markers and often seemed to be incorporated into the walls of tumor lymphatics. Significant p32 expression was common in human cancers and the p32 levels were often greatly elevated compared with the corresponding normal tissue. These results establish p32, particularly its cell-surface-expressed form, as a new marker of tumor cells and tumor-associated macrophages/myeloid cells in hypoxic/metabolically deprived areas of tumors. Its unique localization in tumors and its relative tumor specificity may make p32 a useful target in tumor diagnosis and therapy.
Figures
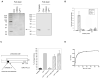
Figure 1. Lyp-1 peptide binds to p32 protein in tumor cell extracts
A. Proteins bound to agarose-linked LyP-1 peptide (CGNKRTRGC) from extracts of cultured MDA-MB-435 cells. Peptides with the sequences CREKA and CRVRTRSGC (CRV) were used as negative controls in the pull down. Left panel: silver staining of LyP-1 bound proteins. The arrow indicates a specific band that was identified as p32 by mass spectrometry. Right panel: Anti-p32 immunoblot of total cell extract (Tot lysate) and proteins bound to the LyP-1 and control peptides. B. Phage binding to p32. Purified p32, or BSA as a control, were coated onto microtiter wells and binding of LyP-1 phage, insertless phage and phage clones displaying the tumor homing peptides CREKA and LyP-2 (CNRRTKAGC) to the wells was tested. Results are expressed as fold of bound peptide phage over insertless phage (±SD) and are representative of five independent experiments. C. Inhibition of LyP-1 phage binding to purified p32 by mAb 60.11. Left panel-Diagrammatic representation of precursor (amino acids 1-282) and mature (amino acids 74-282) forms of p32 protein, shown are also the binding sites for the monoclonal antibodies, mAb 60.11 and mAb 74.5.2. Right panel- Anti-p32 mAb 74.5.2 and purified mouse IgG1 (mIgG; negative control) do not inhibit the binding. The results are representative of three independent experiments and are expressed as percentage of phage binding (±SD), with LyP-1 phage binding alone as 100%. D. Affinity of LyP-1 peptide for purified p32. Binding of increasing amounts of biotinylated LyP-1 peptide to immobilized p32 protein was detected with streptavidin-coupled to horseradish peroxidase and normalized to non-specific binding in the absence of p32. The saturation curve shown is representative of three independent experiments. Error bars indicate SD from triplicate measurements.
Figure 2. LyP-1 binds to p32-expressing cells
A. C8161 cells were transiently transfected with pEGFP together with either empty pCDNA3.1 vector or p32 pCDNA3.1 vector. Transfected cells were sorted for EGFP expression and the sorted populations were used for phage binding assay and immunoblot analysis with anti-p32. LyP-1 phage binding to cells transfected with the empty vector or p32 vector is expressed as fold binding over insertless phage. The graph represents the mean of binding in two independent experiments performed in duplicate (LyP-1 vs insertless phage in p32-transfected cells p< 0.05; Student’s t test). B. MDA-MB-435 S35 cells were transiently transfected with p32-specific or control siRNAs. After 48 hours, inhibition of p32 expression was determined by immunoblot analysis and immunostaining (upper panels). β-actin was used as a control. Lower panels: cells transfected with p32 siRNA or control siRNA were incubated for 1h at 4°C in the presence of 10μM FITC conjugated LyP-1 peptide (lower left) or a control peptide, ARALPSQRSR (ARAL; lower right), which has the same overall charge as LyP-1. Cells incubated in the absence of any peptide served as background control (Blank). Cells with down-regulated p32 expression showed reduced LyP-1 binding relative to control-transfected cells (left panel, red line versus shaded control), whereas their binding to the control peptide was unaffected (right panel, blue line vs shaded control). A representative experiment out of three is shown. C. LyP-1 phage binding in Raji cells in the presence of 80μg/ml of mIgG1 (control), mAb 74.5.2, or increasing amounts (20 to 80μg/ml) of mAb 60.11. Insertless phage was used to determine background phage binding. The results are representative of three independent experiments and are expressed as percentage of phage binding (±SD), with binding of LyP-1 phage in the presence of mIgG1 set as 100%.
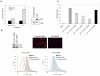
Figure 3. Expression and cell surface localization of p32 in tumor cells
A, C. and D. FACS analysis showing cell surface expression of p32 in tumor cell cultures (A and D, left panel) and single cell suspensions from MDA-MB-435 and C8161 tumor xenografts (C, left panel). Rabbit IgG or a polyclonal antibody against full-length p32 or p32 anti-peptide antibody were applied to live cells and detected with an Alexa 488-labeled secondary antibody. Propidium iodide-negative (living) cells were gated for the analysis. The total expression level of p32 in lysates of the indicated tumor cell lines (B), tumor xenografts (C, right panel) and cells of the MCF-10 series (D, right panel) was detected by immunoblot.
Figure 4. Expression and cell surface localization of p32 in tumors and control organs
A. and B. FACS (A) and immunoblot (B) analyses of cell surface and total expression of p32 in single cell suspensions from MDA-MB-435 (A and B-left panel) or 4T1(B-right panel) tumors, and from the spleen and kidney. C. and D. Affinity purified-polyclonal peptide antibody against p32, or rabbit IgG as a control, were injected into the tail vein of mice bearing MDA-MB-435 (C) or 4T1 (D) tumors. The tumors, spleen and kidneys were removed 4 hour after the injection, sectioned, and examined for the presence of rabbit IgG using Alexa 488 anti-rabbit IgG secondary antibody (green). Nuclei were stained with DAPI (blue).
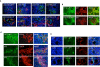
Figure 5. Association of p32 with lymphatic vessels and macrophages in MDA-MB-435 xenograft tumors
A. Double staining of sections from MDA-MB-435 xenograft tumors for p32 (green), lymphatic vessel/macrophage markers podoplanin and LYVE-1, or CD31 and Meca-32 as markers for blood vessels (red). Polyclonal anti-p32 antibody recognizes cell clusters in areas that are positive for podoplanin and/or LYVE-1 (40x magnification). Cells that are positive both for p32 and podoplanin or LYVE-1 frequently line vessel-like structures that are negative for the blood vessel markers (lower panels-60x magnification). B. Upper panels, co-localization of LyP-1 peptide and p32 in tumors. Fluorescein-conjugated LyP-1 peptide was intravenously injected into mice bearing MDA-MB-435 tumors and allowed to circulate for 1 hour. The tumor was then removed for immunohistochemical staining with anti-p32 antibody and analyzed for LyP-1 distribution (green) and p32 expression (red). Lower panels, association of p32 protein with hypoxic areas in tumors. The hypoxia marker pimonidazole hydrochloride (Hypoxyprobe-1) was intravenously injected into mice bearing MDA-MB-435 tumors and let circulate for 1h before tumor removal. Tumor sections were co-stained with anti-Hypoxyprobe-1 (green) and anti-p32 (red) antibodies. C. Partial tumor co-localization of intravenously injected FITC-LyP-1 peptide (upper panel) and p32 protein (lower panels) with the macrophage/myeloid cell markers CD11b and Gr-1. D. Sequential tumor sections were stained separately for CD11b, p32 and podoplanin. Cells integrated into podoplanin-positive vessels bind FITC-LyP-1 and express both CD11b and p32. The images are representative of at least 5 sections examined from each of 10 different tumors.
Figure 6. p32 expression in human tumors
Anti-p32 mAb 60.11 was used to detect p32 in tissue arrays of human tumors and normal tissues by immunohistochemical staining A. Comparison of p32 expression in tumors and the corresponding normal tissues. (magnification 20x) Parallel sections of all tissues examined were incubated with mouse IgG instead of mAb60.11 and showed no staining (not shown). B. Sequential tissue sections were stained separately for epithelial membrane antigen (EMA), p32, and the macrophage/myeloid cells marker CD68. Bar 50μm. C. p32 and CD68 double immunohistochemistry. The tumor array was stained for CD68 (brown) and p32 (purple) and counterstained with Methyl Green. The selected regions (boxes) were subjected to image analysis with Scanscope-HT. The brown and purple colors in the original picture were separated using a color deconvolution algorithm. The separeted colors are shown in brown (CD68) and black (p32). Colocalization of CD68 and p32 are indicated by arrows. Original magnification 40x. (A and B). Examples of breast cancers where p32 is either confined to tumor cells only (1-3) or expressed both in tumor cells and tumor stroma (4-6) are indicated.
Similar articles
- Proapoptotic peptide-mediated cancer therapy targeted to cell surface p32.
Agemy L, Kotamraju VR, Friedmann-Morvinski D, Sharma S, Sugahara KN, Ruoslahti E. Agemy L, et al. Mol Ther. 2013 Dec;21(12):2195-204. doi: 10.1038/mt.2013.191. Epub 2013 Aug 20. Mol Ther. 2013. PMID: 23959073 Free PMC article. - A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection.
Choi Y, Kwon YC, Kim SI, Park JM, Lee KH, Ahn BY. Choi Y, et al. Virology. 2008 Nov 25;381(2):178-83. doi: 10.1016/j.virol.2008.08.035. Epub 2008 Oct 2. Virology. 2008. PMID: 18834607 - The multicompartmental p32/gClqR as a new target for antibody-based tumor targeting strategies.
Sánchez-Martín D, Cuesta AM, Fogal V, Ruoslahti E, Alvarez-Vallina L. Sánchez-Martín D, et al. J Biol Chem. 2011 Feb 18;286(7):5197-203. doi: 10.1074/jbc.M110.161927. Epub 2010 Dec 14. J Biol Chem. 2011. PMID: 21156793 Free PMC article. - The C1q Receptors: Focus on gC1qR/p33 (C1qBP, p32, HABP-1)1.
Ghebrehiwet B, Geisbrecht BV, Xu X, Savitt AG, Peerschke EIB. Ghebrehiwet B, et al. Semin Immunol. 2019 Oct;45:101338. doi: 10.1016/j.smim.2019.101338. Epub 2019 Nov 16. Semin Immunol. 2019. PMID: 31744753 Review. - gC1q-R/p33, a member of a new class of multifunctional and multicompartmental cellular proteins, is involved in inflammation and infection.
Ghebrehiwet B, Lim BL, Kumar R, Feng X, Peerschke EI. Ghebrehiwet B, et al. Immunol Rev. 2001 Apr;180:65-77. doi: 10.1034/j.1600-065x.2001.1800106.x. Immunol Rev. 2001. PMID: 11414365 Review.
Cited by
- Tumor-Targeting Peptides: The Functional Screen of Glioblastoma Homing Peptides to the Target Protein FABP3 (MDGI).
Ayo A, Figueras E, Schachtsiek T, Budak M, Sewald N, Laakkonen P. Ayo A, et al. Cancers (Basel). 2020 Jul 8;12(7):1836. doi: 10.3390/cancers12071836. Cancers (Basel). 2020. PMID: 32650473 Free PMC article. - A Heterotypic Tridimensional Model to Study the Interaction of Macrophages and Glioblastoma In Vitro.
Gattas MJ, Estecho IG, Lago Huvelle MA, Errasti AE, Carrera Silva EA, Simian M. Gattas MJ, et al. Int J Mol Sci. 2021 May 12;22(10):5105. doi: 10.3390/ijms22105105. Int J Mol Sci. 2021. PMID: 34065977 Free PMC article. - High-Throughput Approaches to the Development of Molecular Imaging Agents.
Hu LY, Kelly KA, Sutcliffe JL. Hu LY, et al. Mol Imaging Biol. 2017 Apr;19(2):163-182. doi: 10.1007/s11307-016-1016-z. Mol Imaging Biol. 2017. PMID: 27812924 Review. - Complement C1q Binding Protein (C1QBP): Physiological Functions, Mutation-Associated Mitochondrial Cardiomyopathy and Current Disease Models.
Wang J, Huang CL, Zhang Y. Wang J, et al. Front Cardiovasc Med. 2022 Mar 2;9:843853. doi: 10.3389/fcvm.2022.843853. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35310974 Free PMC article. Review. - Connecting Metabolic Rewiring With Phenotype Switching in Melanoma.
Falletta P, Goding CR, Vivas-García Y. Falletta P, et al. Front Cell Dev Biol. 2022 Jul 15;10:930250. doi: 10.3389/fcell.2022.930250. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912100 Free PMC article. Review.
References
- Croix B, St, Rago C, Velculescu V, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–202. - PubMed
- Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. - PubMed
- Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat Med. 2002;8:751–5. - PubMed
- Zhang L, Giraudo E, Hoffman JA, Hanahan D, Ruoslahti E. Lymphatic zip codes in premalignant lesions and tumors. Cancer Res. 2006;66:5696–706. - PubMed
- Jain RK. The next frontier of molecular medicine: delivery of therapeutics. Nat Med. 1998;4:655–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA 30199/CA/NCI NIH HHS/United States
- CA115410/CA/NCI NIH HHS/United States
- R01 CA115410/CA/NCI NIH HHS/United States
- P01 CA104898/CA/NCI NIH HHS/United States
- P30 CA030199/CA/NCI NIH HHS/United States
- CA104898/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials