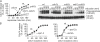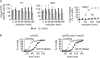Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase - PubMed (original) (raw)
Separase cooperates with Zds1 and Zds2 to activate Cdc14 phosphatase in early anaphase
Ethel Queralt et al. J Cell Biol. 2008.
Abstract
Completion of mitotic exit and cytokinesis requires the inactivation of mitotic cyclin-dependent kinase (Cdk) activity. A key enzyme that counteracts Cdk during budding yeast mitotic exit is the Cdc14 phosphatase. Cdc14 is inactive for much of the cell cycle, sequestered by its inhibitor Net1 in the nucleolus. At anaphase onset, separase-dependent down-regulation of PP2A(Cdc55) allows phosphorylation of Net1 and consequent Cdc14 release. How separase causes PP2A(Cdc55) down-regulation is not known. Here, we show that two Cdc55-interacting proteins, Zds1 and Zds2, contribute to timely Cdc14 activation during mitotic exit. Zds1 and Zds2 are required downstream of separase to facilitate nucleolar Cdc14 release. Ectopic Zds1 expression in turn is sufficient to down-regulate PP2A(Cdc55) and promote Net1 phosphorylation. These findings identify Zds1 and Zds2 as new components of the mitotic exit machinery, involved in activation of the Cdc14 phosphatase at anaphase onset. Our results suggest that these proteins may act as separase-regulated PP2A(Cdc55) inhibitors.
Figures
Figure 1.
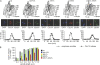
Zds1 and Zds2 interact with PP2ACdc55. Cell extracts were prepared from strains Y2541 (_MAT_a GAL-CDC20 HA3-CDC55), Y3131 (as Y2541, but also ZDS1-Pk9), and Y3200 (as Y2541, but also ZDS2-Pk9), which were synchronized at the metaphase to anaphase transition, and coimmunoprecipitation of subunits of the PP2ACdc55 complex with Zds1 and Zds2 was analyzed by Western blotting. The apparent molecular weights of the subunits, relative to marker proteins during gel electrophoresis, are indicated. Anaphase progression was monitored by tubulin staining.
Figure 2.
Delayed Cdc14 nucleolar release in the absence of Zds1 and Zds2. (A) Analysis of mitotic progression in the absence of Zds1 and Zds2. Strains Y2786 (_MAT_a CDC14-Pk9), Y1016 (as Y2786, but spo12Δ), Y3013 (as Y2786, but zds1Δ), Y3036 (as Y2786, but zds2Δ), and Y3117 (as Y2786, but zds1Δ zds2Δ) were arrested with α-factor and released into a synchronous cell cycle. FACS analysis of DNA content was used to monitor cell cycle progression. Cdc14 nucleolar release and anaphase spindle elongation was analyzed by in situ immunofluorescence. At least 100 cells were scored at each time point. Photographs are shown of cells in early anaphase with a spindle length of 3–5 μm. Bar, 5 μm. (B) Cdc14 nucleolar release was analyzed in samples of the experiment in A as a function of spindle length.
Figure 3.
Zds1 promotes Cdc14 nucleolar release by Net1 phosphorylation. (A) Ectopic expression of Zds1 and Zds2 promote Cdc14 nucleolar release. Strains Y3288 (_MAT_a MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9) and Y2220 (_MAT_a MET-CDC20 GAL-Flag-ZDS2 CDC14-Pk9) were arrested in metaphase (meta) and Zds1 or Zds2 expression induced. Part of the culture of strain Y3288 was maintained without Zds1 induction during the course of the experiment. Cdc14 nucleolar release was monitored by immunofluorescence, and Zds1 and Zds2 expression levels were analyzed by Western blotting. Tubulin served as a loading control. Photographs are shown of cells in metaphase and 120 min after Zds1 or Zds2 induction. Bar, 5 μm. (B) Cdk phosphorylation sites are required for Zds1-induced Net1 phosphorylation and Cdc14 release. Strains Y1008 (_MAT_a MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9 NET1-myc9) and Y1013 (as Y1008, but net1-6Cdk-myc9) were arrested in metaphase by Cdc20 depletion and Zds1 expression was induced. Net1 phosphorylation was monitored by Western blotting against the myc epitope. Apparent relative molecular weights are indicated.
Figure 4.
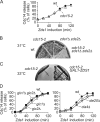
Zds1 and the MEN. (A) The essential MEN kinase Cdc15 is not required for Zds1-induced Cdc14 release. Strains Y3288 (_MAT_a MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9) and Y1429 (as Y3288, but cdc15-2) were arrested in metaphase by Cdc20 depletion and shifted to 37°C for 60 min before Zds1 induction. (B) cdc15-2 and zds1Δ zds2Δ mutations display a synthetic growth defect. Strains Y699 (W303), Y3196 (_MAT_a cdc15-2), Y3117 (_MAT_a zds1Δ zds2Δ), and Y3428 (as Y3117, but cdc15-2) were streaked onto YPD plates and incubated at 31°C for 2 d. (C) Zds1 overexpression rescues growth of cdc15-2 cells at restrictive temperature. Strains Y3196 and Y3191 (_MAT_a cdc15-2 GAL1-ZDS1) were streaked onto YP plates containing raffinose and galactose, to induce Zds1 expression, at 33°C for 2 d. (D) Cdc42 effectors and PAK-kinases are not required for Zds1-induced Cdc14 release. Strains Y3288 (_MAT_a MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9), Y1409 (as Y3288, but ste20Δ), Y1410 (as Y3288, but cla4Δ), Y1412 (as Y3288, but gic1Δ), Y1411 (as Y3288, but gic2Δ), and Y1414 (as Y3288, but gic1Δ gic2Δ) were arrested in metaphase by Cdc20 depletion and Zds1 expression was induced. Cdc14 nucleolar release was monitored by immunofluorescence. Equal Zds1 expression levels were confirmed by Western blotting (not depicted).
Figure 5.
Zds1 is required for separase-induced Cdc14 early anaphase release. (A) Zds1 and Zds2 are required for separase-induced Cdc14 release. Strains Y1748 (_MAT_a GAL-ESP1-HA CDC14-Pk9), Y1790 (as Y1748, but zds1Δ), Y1959 (as Y1748, but zds2Δ), and Y2189 (as Y1790, but zds1Δ zds2Δ) were arrested in metaphase by nocodazole treatment and separase expression induced. Separase levels were analyzed by Western blotting, using Cdc28 as loading control. (B) Separase, Slk19, and Spo12 are not required for Zds1-induced Cdc14 release. Strains Y3288 (_MAT_a MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9) and Y1430 (as Y3288, but esp1-2) were arrested in metaphase by Cdc20 depletion at 23°C and shifted to 37°C for 60 min before Zds1 induction. Strains Y3288 (_MAT_a MET-CDC20 GAL-Flag-ZDS1 CDC14-Pk9), Y1521 (as Y3288, but slk19Δ), and Y1646 (as Y3288, but spo12Δ) were arrested in metaphase by Cdc20 depletion at 23°C and Zds1 expression was induced.
Figure 6.
Zds1 promotes PP2ACdc55 down-regulation. (A) Zds1 causes down-regulation of PP2ACdc55 phosphatase activity. Strains Y2627 (_MAT_a MET-CDC20 HA3-CDC55 CDC14-myc18) and Y1015 (as Y2627, but GAL-Flag-ZDS1 CDC14-Pk9) were arrested in metaphase by Cdc20 depletion and galactose added to induce Zds1 expression. The specific phosphatase activity of immunopurified Cdc55 was measured as described in Materials and methods. Mean and standard deviation of the specific phosphatase activity relative to metaphase in three experiments are shown. Cdc14 nucleolar release in these experiments was analyzed in parallel. (B) Zds1 and Zds2 are dispensable for early Cdc14 nucleolar release in the absence of Cdc55. Strains Y2831 (_MAT_a cdc55Δ CDC14-Pk9) and Y3419 (as 2831, but zds1Δ zds2Δ) were synchronized in G1 by pheromone α-factor treatment and released to progress through the cell cycle into nocodazole-imposed metaphase arrest. The budding index and Cdc14 nucleolar release were determined.
Figure 7.
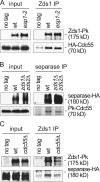
Interactions between separase, Zds1, and Cdc55. (A) Zds1 and Cdc55 interact independently of separase. Co-immunoprecipitation between Zds1 and Cdc55 was analyzed in protein extracts from strains Y585 (_MAT_a Zds1-Pk6 HA3-CDC55) and Y586 (as 585, but esp1-2) that were grown at 33°C, a restrictive temperature for the separase esp1-2 allele, for 1 h before extract preparation. Protein extracts from strain Y584 (_MAT_a HA3-CDC55) lacking a Pk epitope on Zds1 served as a control. (B) Separase and Cdc55 interact independently of Zds1 and Zds2. (B) As (A), but Cdc55 co-immuoprecipitation with separase from extracts of strains Y587 (_MAT_a ESP1-HA6), Y3434 (as 587, but Pk3-CDC55), and Y3427 (as 3434, but zds1Δ zds2Δ) grown at 25°C was analyzed. (C) An interaction between separase and Zds1. Co-immunoprecipitation of separase with Zds1 was analyzed in extracts from strains Y587 (_MAT_a ESP1-HA6), Y588 (as 587, but Zds1-Pk6), and Y589 (as 588, but cdc55Δ).
Similar articles
- Zds1 regulates PP2A(Cdc55) activity and Cdc14 activation during mitotic exit through its Zds_C motif.
Calabria I, Baro B, Rodriguez-Rodriguez JA, Russiñol N, Queralt E. Calabria I, et al. J Cell Sci. 2012 Jun 15;125(Pt 12):2875-84. doi: 10.1242/jcs.097865. Epub 2012 Mar 16. J Cell Sci. 2012. PMID: 22427694 Free PMC article. - Cdc14 activation requires coordinated Cdk1-dependent phosphorylation of Net1 and PP2A-Cdc55 at anaphase onset.
Játiva S, Calabria I, Moyano-Rodriguez Y, Garcia P, Queralt E. Játiva S, et al. Cell Mol Life Sci. 2019 Sep;76(18):3601-3620. doi: 10.1007/s00018-019-03086-5. Epub 2019 Mar 29. Cell Mol Life Sci. 2019. PMID: 30927017 Free PMC article. - Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast.
Queralt E, Lehane C, Novak B, Uhlmann F. Queralt E, et al. Cell. 2006 May 19;125(4):719-32. doi: 10.1016/j.cell.2006.03.038. Cell. 2006. PMID: 16713564 - Complexity of mitotic exit.
Jensen S, Johnston LH. Jensen S, et al. Cell Cycle. 2002 Sep-Oct;1(5):300-3. doi: 10.4161/cc.1.5.142. Cell Cycle. 2002. PMID: 12461287 Review. - Cdc14 and the temporal coordination between mitotic exit and chromosome segregation.
Torres-Rosell J, Machín F, Aragón L. Torres-Rosell J, et al. Cell Cycle. 2005 Jan;4(1):109-12. doi: 10.4161/cc.4.1.1356. Epub 2005 Jan 10. Cell Cycle. 2005. PMID: 15611663 Review.
Cited by
- The RSC chromatin-remodeling complex influences mitotic exit and adaptation to the spindle assembly checkpoint by controlling the Cdc14 phosphatase.
Rossio V, Galati E, Ferrari M, Pellicioli A, Sutani T, Shirahige K, Lucchini G, Piatti S. Rossio V, et al. J Cell Biol. 2010 Nov 29;191(5):981-97. doi: 10.1083/jcb.201007025. Epub 2010 Nov 22. J Cell Biol. 2010. PMID: 21098112 Free PMC article. - Regulation of Spo12 phosphorylation and its essential role in the FEAR network.
Tomson BN, Rahal R, Reiser V, Monje-Casas F, Mekhail K, Moazed D, Amon A. Tomson BN, et al. Curr Biol. 2009 Mar 24;19(6):449-60. doi: 10.1016/j.cub.2009.02.024. Epub 2009 Mar 5. Curr Biol. 2009. PMID: 19268588 Free PMC article. - The Zds proteins control entry into mitosis and target protein phosphatase 2A to the Cdc25 phosphatase.
Wicky S, Tjandra H, Schieltz D, Yates J 3rd, Kellogg DR. Wicky S, et al. Mol Biol Cell. 2011 Jan 1;22(1):20-32. doi: 10.1091/mbc.E10-06-0487. Epub 2010 Nov 30. Mol Biol Cell. 2011. PMID: 21119008 Free PMC article. - Budding yeast greatwall and endosulfines control activity and spatial regulation of PP2A(Cdc55) for timely mitotic progression.
Juanes MA, Khoueiry R, Kupka T, Castro A, Mudrak I, Ogris E, Lorca T, Piatti S. Juanes MA, et al. PLoS Genet. 2013;9(7):e1003575. doi: 10.1371/journal.pgen.1003575. Epub 2013 Jul 4. PLoS Genet. 2013. PMID: 23861665 Free PMC article. - Zds1 regulates PP2A(Cdc55) activity and Cdc14 activation during mitotic exit through its Zds_C motif.
Calabria I, Baro B, Rodriguez-Rodriguez JA, Russiñol N, Queralt E. Calabria I, et al. J Cell Sci. 2012 Jun 15;125(Pt 12):2875-84. doi: 10.1242/jcs.097865. Epub 2012 Mar 16. J Cell Sci. 2012. PMID: 22427694 Free PMC article.
References
- Azzam, R., S.L. Chen, W. Shou, A.S. Mah, G. Alexandru, K. Nasmyth, R.S. Annan, S.A. Carr, and R.J. Deshaies. 2004. Phosphorylation by cyclin B-Cdk underlies release of mitotic exit activator Cdc14 from the nucleolus. Science. 305:516–519. - PubMed
- Bardin, A.J., R. Visintin, and A. Amon. 2000. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 102:21–31. - PubMed
- Bishop, A.C., J.A. Ubersax, D.T. Petsch, D.P. Matheos, N.S. Gray, J. Blethrow, E. Shimizu, J.Z. Tsien, P.G. Schultz, M.D. Rose, et al. 2000. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 407:395–401. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases