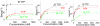Selecting aptamers for a glycoprotein through the incorporation of the boronic acid moiety - PubMed (original) (raw)
. 2008 Sep 24;130(38):12636-8.
doi: 10.1021/ja801510d. Epub 2008 Sep 3.
Affiliations
- PMID: 18763762
- PMCID: PMC2655352
- DOI: 10.1021/ja801510d
Selecting aptamers for a glycoprotein through the incorporation of the boronic acid moiety
Minyong Li et al. J Am Chem Soc. 2008.
Abstract
The first general method for the selection of boronic acid-based aptamers (boronolectins) that allows for glycan substructure focusing is described. Using fibrinogen as a model glycoprotein, we have selected boronic acid-modified DNA aptamers that have high affinities (low nM Kd) and the ability to recognize changes in the glycosylation site. The method developed should also be applicable to the development of aptamers for other glycoproducts, such as glycolipids and glycopeptides.
Figures
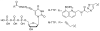
Figure 1
The chemical structures of B-TTP and M-TTP
Scheme 1
SELEX scheme for selecting DNA aptamers with high affinity for fibrinogen
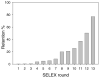
Figure 2
Retention of radioactive DNA on fibrinogen-immobilized beads during 13 rounds of selection
Figure 3
Binding curves of B-TTP-, TTP- and M-TTP-derived 85A with fibrinogen (left), deglycosylated fibrinogen (middle) and periodated fibrinogen (right)
Similar articles
- Glycan-Imprinted Magnetic Nanoparticle-Based SELEX for Efficient Screening of Glycoprotein-Binding Aptamers.
Ma Y, Li X, Li W, Liu Z. Ma Y, et al. ACS Appl Mater Interfaces. 2018 Nov 28;10(47):40918-40926. doi: 10.1021/acsami.8b14441. Epub 2018 Nov 13. ACS Appl Mater Interfaces. 2018. PMID: 30379519 - Efficient selection of glycoprotein-binding DNA aptamers via boronate affinity monolithic capillary.
Nie H, Chen Y, Lü C, Liu Z. Nie H, et al. Anal Chem. 2013 Sep 3;85(17):8277-83. doi: 10.1021/ac4015353. Epub 2013 Aug 12. Anal Chem. 2013. PMID: 23895515 - A sol-gel-based microfluidics system enhances the efficiency of RNA aptamer selection.
Ahn JY, Jo M, Dua P, Lee DK, Kim S. Ahn JY, et al. Oligonucleotides. 2011 Mar-Apr;21(2):93-100. doi: 10.1089/oli.2010.0263. Epub 2011 Mar 17. Oligonucleotides. 2011. PMID: 21413890 - SELEX--a (r)evolutionary method to generate high-affinity nucleic acid ligands.
Stoltenburg R, Reinemann C, Strehlitz B. Stoltenburg R, et al. Biomol Eng. 2007 Oct;24(4):381-403. doi: 10.1016/j.bioeng.2007.06.001. Epub 2007 Jun 16. Biomol Eng. 2007. PMID: 17627883 Review. - Methods for selection of aptamers to protein targets.
Kulbachinskiy AV. Kulbachinskiy AV. Biochemistry (Mosc). 2007 Dec;72(13):1505-18. doi: 10.1134/s000629790713007x. Biochemistry (Mosc). 2007. PMID: 18282139 Review.
Cited by
- Nucleic Acid Aptamers: An Emerging Tool for Biotechnology and Biomedical Sensing.
Ku TH, Zhang T, Luo H, Yen TM, Chen PW, Han Y, Lo YH. Ku TH, et al. Sensors (Basel). 2015 Jul 6;15(7):16281-313. doi: 10.3390/s150716281. Sensors (Basel). 2015. PMID: 26153774 Free PMC article. Review. - Multivalent glycocluster design through directed evolution.
MacPherson IS, Temme JS, Habeshian S, Felczak K, Pankiewicz K, Hedstrom L, Krauss IJ. MacPherson IS, et al. Angew Chem Int Ed Engl. 2011 Nov 18;50(47):11238-42. doi: 10.1002/anie.201105555. Angew Chem Int Ed Engl. 2011. PMID: 22191092 Free PMC article. No abstract available. - Rational evolution of Cd2+-specific DNAzymes with phosphorothioate modified cleavage junction and Cd2+ sensing.
Huang PJ, Liu J. Huang PJ, et al. Nucleic Acids Res. 2015 Jul 13;43(12):6125-33. doi: 10.1093/nar/gkv519. Epub 2015 May 18. Nucleic Acids Res. 2015. PMID: 25990730 Free PMC article. - Functional nucleic acid sensors.
Liu J, Cao Z, Lu Y. Liu J, et al. Chem Rev. 2009 May;109(5):1948-98. doi: 10.1021/cr030183i. Chem Rev. 2009. PMID: 19301873 Free PMC article. Review. No abstract available. - Trends in the Design and Development of Specific Aptamers Against Peptides and Proteins.
Tabarzad M, Jafari M. Tabarzad M, et al. Protein J. 2016 Apr;35(2):81-99. doi: 10.1007/s10930-016-9653-2. Protein J. 2016. PMID: 26984473 Review.
References
- Fukuda M. In: Molecular Glycobiology. Fukuda M, Hindsgaul O, editors. Oxford University Press; New York: 1994. pp. 1–52.
- Klussmann S. The Aptamer Handbook-Functional Oligonucleotides and Their Applications. Wiley-VCH Werlag GMbH & Co. KGaA; Weinheim: 2006. Famulok M, Hartig JS, Mayer G. Chem. Rev. 2007;107:3715–3743. and references cited therein.
- Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2005. Manimala JC, Wiskur SL, Ellington AD, Anslyn EV. J. Am. Chem. Soc. 2004;126:16515.James TD, Shinkai S. Top. Curr. Chem. 2002;218:159–200.Springsteen G, Wang B. Tetrahedron. 2002;58:5291–5300.Wang W, Gao X, Wang B. Curr. Org. Chem. 2002;6:1285–1317.Yan J, Fang H, Wang B. Med. Res. Rev. 2005;25:490–520. and references cited therein.
- Groll M, Berkers CR, Ploegh HL, Ovaa H. Structure. 2006;14:451–456.Yang W, Gao X, Wang B. Med. Res. Rev. 2003;23:346–368. and references cited therein.
Publication types
MeSH terms
Substances
Grants and funding
- R21 CA113917-02S1/CA/NCI NIH HHS/United States
- CA113917/CA/NCI NIH HHS/United States
- R21 CA113917-02/CA/NCI NIH HHS/United States
- R21 CA113917/CA/NCI NIH HHS/United States
- GM084933/GM/NIGMS NIH HHS/United States
- R21 CA113917-01/CA/NCI NIH HHS/United States
- R21 CA123329-01/CA/NCI NIH HHS/United States
- R01 GM084933/GM/NIGMS NIH HHS/United States
- R21 CA113917-01S1/CA/NCI NIH HHS/United States
- R21 CA123329-02/CA/NCI NIH HHS/United States
- R01 GM084933-01/GM/NIGMS NIH HHS/United States
- R21 CA123329/CA/NCI NIH HHS/United States
- CA123329/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources