A PDZ-binding motif controls basolateral targeting of syndecan-1 along the biosynthetic pathway in polarized epithelial cells - PubMed (original) (raw)
A PDZ-binding motif controls basolateral targeting of syndecan-1 along the biosynthetic pathway in polarized epithelial cells
Sandra Maday et al. Traffic. 2008 Nov.
Abstract
The cell surface proteoglycan, syndecan-1, is essential for normal epithelial morphology and function. Syndecan-1 is selectively localized to the basolateral domain of polarized epithelial cells and interacts with cytosolic PDZ (PSD-95, discs large, ZO-1) domain-containing proteins. Here, we show that the polarity of syndecan-1 is determined by its type II PDZ-binding motif. Mutations within the PDZ-binding motif lead to the mislocalization of syndecan-1 to the apical surface. In contrast to previous examples, however, PDZ-binding motif-dependent polarity is not determined by retention at the basolateral surface but rather by polarized sorting prior to syndecan-1's arrival at the plasma membrane. Although none of the four known PDZ-binding partners of syndecan-1 appears to control basolateral localization, our results show that the PDZ-binding motif of syndecan-1 is decoded along the biosynthetic pathway establishing a potential role for PDZ-mediated interactions in polarized sorting.
Figures
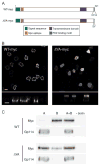
Figure 1. Truncation of the PDZ-binding motif causes apical mislocalization of syndecan-1
A) Polarized MDCK cells stably expressing WT syndecan-1 were fixed, permeabilized, processed for IF for syndecan-1 and gp58, and viewed by confocal microscopy. En face images and X–Z sections are shown. Bars, 10 μm. B) Sequence of the cytoplasmic tail of syndecan-1, with the PDZ-binding motif (blue). The last two residues were deleted in ΔYA. C) Polarized MDCK cells stably expressing ΔYA syndecan-1 were processed as in A. Bars, 10 μm.
Figure 2. Biochemical characterization of ΔYA myc-syndecan-1 mislocalization
A) An myc tag was introduced at the N-terminus, after amino acid 22, five residues after the predicted 17-amino acid signal sequence. B and C) Polarized MDCK cells were infected with adenoviruses encoding either WT or ΔYA myc-syndecan-1 for 20 h. B) Cells were surface labeled for syndecan-1, processed for IF and analyzed by confocal microscopy. Bars, 10 μm. C) Cells were placed on ice and biotin was added to the apical domain (A), the basolateral domain (B) or both the apical and basolateral domains (A+B). Biotin was not added in one sample as a control. Biotinylated domain-specific surface protein was retrieved and processed for immunoblot.
Figure 3. Additional mutations within the PDZ-binding motif cause apical mislocalization
A) Polarized MDCK cells stably expressing the indicated syndecan-1 mutant were surface labeled for syndecan-1, processed for IF and analyzed by confocal microscopy. Bars, 10 μm. B) Polarized MDCK cells stably expressing the indicated syndecan-1 mutant were placed on ice and biotin was added to the apical (A) or basolateral (B) domain. Samples were processed as in Figure 2C and quantified in C. Data represent mean values from two independent experiments and error bars indicate standard deviation.
Figure 4. PDZ-binding motif targets syndecan-1 directly to the basolateral membrane
A) Polarized MDCK cells were infected with adenoviruses encoding WT or ΔYA myc-syndecan-1 for 20 h. Cells were pulsed for 15 min at 37°C with 2 mCi/ml 35S-methionine and 35S-cysteine, and chased for 0–3 h. At each time point, cells were placed on ice and biotinylated on the apical (A) or basolateral (B) domain. Total syndecan-1 protein was immunopre-cipitated and eluted. Biotinylated domain-specific surface syndecan-1 protein was retrieved and processed for SDS–PAGE and autoradiography. B) Quantification of A. Data represent mean values from two independent experiments and error bars indicate standard deviation.
Similar articles
- GOPC facilitates the sorting of syndecan-1 in polarized epithelial cells.
Ford C, Burd CG. Ford C, et al. Mol Biol Cell. 2022 Sep 1;33(10):ar86. doi: 10.1091/mbc.E22-05-0165. Epub 2022 Jul 13. Mol Biol Cell. 2022. PMID: 35830596 Free PMC article. - PDZ-domain-directed basolateral targeting of the peripheral membrane protein FRMPD2 in epithelial cells.
Stenzel N, Fetzer CP, Heumann R, Erdmann KS. Stenzel N, et al. J Cell Sci. 2009 Sep 15;122(Pt 18):3374-84. doi: 10.1242/jcs.046854. Epub 2009 Aug 25. J Cell Sci. 2009. PMID: 19706687 - PDZ-mediated interactions retain the epithelial GABA transporter on the basolateral surface of polarized epithelial cells.
Perego C, Vanoni C, Villa A, Longhi R, Kaech SM, Fröhli E, Hajnal A, Kim SK, Pietrini G. Perego C, et al. EMBO J. 1999 May 4;18(9):2384-93. doi: 10.1093/emboj/18.9.2384. EMBO J. 1999. PMID: 10228153 Free PMC article. - PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes.
Brône B, Eggermont J. Brône B, et al. Am J Physiol Cell Physiol. 2005 Jan;288(1):C20-9. doi: 10.1152/ajpcell.00368.2004. Am J Physiol Cell Physiol. 2005. PMID: 15591244 Review. - Role of the PDZ scaffolding protein in tubule cells in maintenance of polarised function.
Glynne PA, Evans TJ. Glynne PA, et al. Exp Nephrol. 2002;10(5-6):307-12. doi: 10.1159/000065307. Exp Nephrol. 2002. PMID: 12381914 Review.
Cited by
- The Role and Therapeutic Value of Syndecan-1 in Cancer Metastasis and Drug Resistance.
Guo S, Wu X, Lei T, Zhong R, Wang Y, Zhang L, Zhao Q, Huang Y, Shi Y, Wu L. Guo S, et al. Front Cell Dev Biol. 2022 Jan 18;9:784983. doi: 10.3389/fcell.2021.784983. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35118073 Free PMC article. Review. - Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia.
Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, Philp NJ. Castorino JJ, et al. Traffic. 2011 Apr;12(4):483-98. doi: 10.1111/j.1600-0854.2010.01155.x. Epub 2011 Feb 1. Traffic. 2011. PMID: 21199217 Free PMC article. - The Tiam1 PDZ domain couples to Syndecan1 and promotes cell-matrix adhesion.
Shepherd TR, Klaus SM, Liu X, Ramaswamy S, DeMali KA, Fuentes EJ. Shepherd TR, et al. J Mol Biol. 2010 May 21;398(5):730-46. doi: 10.1016/j.jmb.2010.03.047. Epub 2010 Mar 31. J Mol Biol. 2010. PMID: 20361982 Free PMC article. - Mutation conferring apical-targeting motif on AE1 exchanger causes autosomal dominant distal RTA.
Fry AC, Su Y, Yiu V, Cuthbert AW, Trachtman H, Karet Frankl FE. Fry AC, et al. J Am Soc Nephrol. 2012 Jul;23(7):1238-49. doi: 10.1681/ASN.2012020112. Epub 2012 Apr 19. J Am Soc Nephrol. 2012. PMID: 22518001 Free PMC article. - An Atlas of Heparan Sulfate Proteoglycans in the Postnatal Rat Lens.
Wishart TFL, Lovicu FJ. Wishart TFL, et al. Invest Ophthalmol Vis Sci. 2021 Nov 1;62(14):5. doi: 10.1167/iovs.62.14.5. Invest Ophthalmol Vis Sci. 2021. PMID: 34730792 Free PMC article.
References
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01CA461285/CA/NCI NIH HHS/United States
- R01GM29765/GM/NIGMS NIH HHS/United States
- K08 DK059341-05/DK/NIDDK NIH HHS/United States
- K08DK059341/DK/NIDDK NIH HHS/United States
- K08 DK059341/DK/NIDDK NIH HHS/United States
- R01 GM029765/GM/NIGMS NIH HHS/United States
- P01 CA046128/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources