Directional sensitivity of the retina: 75 years of Stiles-Crawford effect - PubMed (original) (raw)
Review
Directional sensitivity of the retina: 75 years of Stiles-Crawford effect
Gerald Westheimer. Proc Biol Sci. 2008.
Abstract
The reduction of the brightness when a light beam's entry into the eye is shifted from the centre to the edge of the pupil has from the outset been shown to be due to a change in luminous efficiency of radiation when it is incident obliquely on the retina. The phenomenon is most prominent in photopic vision and this has concentrated attention on the properties of retinal cones, where responsibility has yet to be assigned to factors such as differences in shape, fine structure and configuration, and membrane anchoring of photopigment molecules. Geometrical optics and waveguide formulations have been applied to the question of how light is guided in receptors, but details of their geometry and optical parameters even if they become available will make calculations complex and of only moderate generality. In practice, the diminution of oblique light helps visual performance by reducing deleterious influence of ocular aberrations and of glare caused by light scattering when the pupil is wide. Receptor orientation can come into play in ocular conditions due to mechanical disturbance and has been shown to have potentiality as a tool for clinical diagnosis. Currently, open questions include microanatomical and molecular differences between rods and cones, the coupling of the optical image of the eye with the transducing apparatus in the photoreceptors, possible phototropism and more convincing methods of estimating the actual spatial distribution of photon events as it affects visual resolution.
Figures
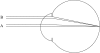
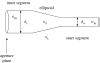
Figure 1
Schematic eye with a wide pupil showing two identical bundles of rays from a distant object focused on the retina that enter through different pupillary regions: A, the centre, and B, near the edge. Stiles & Crawford (1933) discovered that, in photopic vision, the oblique incidence of beam B causes a prominent diminution of luminous efficiency, since called the Stiles–Crawford effect.
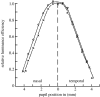
Figure 2
Relative luminance efficiency for narrow bundles, as a function of pupil location of the entering beam. Data for two sets of measurements three months apart in the left eye of W. S. Stiles (Stiles & Crawford 1933).
Figure 3
Microscopic view of a (a) mammalian cone and (b) rod, somewhat schematized, illustrating that morphology could account for the difference in acceptance of obliquely incident light. For cones, the width of inner segments and the width, length and taper of outer segments can vary several-fold depending on the location. Foveal cones have a rod-like appearance; in the retinal periphery, wide, stubby cones are intermixed with rods. Phototransduction takes place in the outer segment. Adapted with permission from Borwein (1981).
Figure 4
Nineteenth-century drawing (Greeff 1874) of cones in three locations in the human retina. (a) In extreme periphery; (b) in 30° periphery; (c) in fovea.
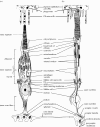
Figure 5
Schematic of high-magnification histological sections of mammalian (a) rod and (b) cone outer segments showing the membraneous invaginations of cones (a detailed view in the inset) and the intracellular stacked discs of rods in which the photopigment molecules are embedded. Adapted from Oyster (1999).
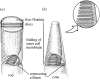
Figure 6
A model of a cone receptor used for geometrical ray tracing and for computation of waveguide modes. The dimension of the diameters _d_i and _d_o and of the lengths of the inner and outer segments and the taper of the ellipsoid are taken from anatomical measurements. The refractive index values of the intra-receptor regions and the extracellular space are estimates from mammalian biological preparations. Apart from the schematized geometrical shape, the important feature of the model is that light is accepted only at one aperture and is guided from there into the interior space of the receptor.
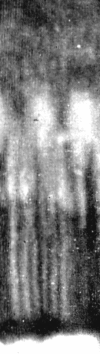
Figure 7
A photomicrograph of a small section of the parafoveal mixed rod–cone retina in a macaque, especially prepared to preserve as best as possible the in vivo orientation of the receptors. Reprinted with permission from Laties (1969).
Similar articles
- Volumetric integration model of the Stiles-Crawford effect of the first kind and its experimental verification.
Vohnsen B, Carmichael A, Sharmin N, Qaysi S, Valente D. Vohnsen B, et al. J Vis. 2017 Oct 1;17(12):18. doi: 10.1167/17.12.18. J Vis. 2017. PMID: 29090313 - Dependence of the magnitude of the Stiles-Crawford effect on retinal location.
Westheimer G. Westheimer G. J Physiol. 1967 Sep;192(2):309-15. doi: 10.1113/jphysiol.1967.sp008301. J Physiol. 1967. PMID: 6050150 Free PMC article. - Vertebrate receptor optics and orientation.
Enoch JM. Enoch JM. Doc Ophthalmol. 1980 Apr 15;48(2):373-88. doi: 10.1007/BF00141466. Doc Ophthalmol. 1980. PMID: 6995055 Review. - Wavelength dependence of the Stiles-Crawford effect explained by perception of backscattered light from the choroid.
Berendschot TJ, van de Kraats J, van Norren D. Berendschot TJ, et al. J Opt Soc Am A Opt Image Sci Vis. 2001 Jul;18(7):1445-51. doi: 10.1364/josaa.18.001445. J Opt Soc Am A Opt Image Sci Vis. 2001. PMID: 11444534 - [Physiology of the visual retinal signal: From phototransduction to the visual cycle].
Salesse C. Salesse C. J Fr Ophtalmol. 2017 Mar;40(3):239-250. doi: 10.1016/j.jfo.2016.12.006. Epub 2017 Mar 17. J Fr Ophtalmol. 2017. PMID: 28318721 Review. French.
Cited by
- Measurement of the photoreceptor pointing in the living chick eye.
Walker MK, Blanco L, Kivlin R, Choi SS, Doble N. Walker MK, et al. Vision Res. 2015 Apr;109(Pt A):59-67. doi: 10.1016/j.visres.2015.01.025. Epub 2015 Feb 23. Vision Res. 2015. PMID: 25722105 Free PMC article. - Modal content of living human cone photoreceptors.
Liu Z, Kocaoglu OP, Turner TL, Miller DT. Liu Z, et al. Biomed Opt Express. 2015 Aug 17;6(9):3378-404. doi: 10.1364/BOE.6.003378. eCollection 2015 Sep 1. Biomed Opt Express. 2015. PMID: 26417509 Free PMC article. - Differential detection of retinal directionality.
Qaysi S, Valente D, Vohnsen B. Qaysi S, et al. Biomed Opt Express. 2018 Nov 16;9(12):6318-6330. doi: 10.1364/BOE.9.006318. eCollection 2018 Dec 1. Biomed Opt Express. 2018. PMID: 31065431 Free PMC article. - A pupillary contrast response in mice and humans: Neural mechanisms and visual functions.
Fitzpatrick MJ, Krizan J, Hsiang JC, Shen N, Kerschensteiner D. Fitzpatrick MJ, et al. Neuron. 2024 Jul 17;112(14):2404-2422.e9. doi: 10.1016/j.neuron.2024.04.012. Epub 2024 May 1. Neuron. 2024. PMID: 38697114 - Honeycomb-shaped electro-neural interface enables cellular-scale pixels in subretinal prosthesis.
Flores T, Huang T, Bhuckory M, Ho E, Chen Z, Dalal R, Galambos L, Kamins T, Mathieson K, Palanker D. Flores T, et al. Sci Rep. 2019 Jul 23;9(1):10657. doi: 10.1038/s41598-019-47082-y. Sci Rep. 2019. PMID: 31337815 Free PMC article.
References
- Applegate R.A, Bonds A.B. Induced movement of receptor alignment towards a new pupillary aperture. Invest. Ophthalmol. Vis. Sci. 1981;21:869–873. - PubMed
- Barer R. Refractometry and interferometry of living cells. J. Opt. Soc. Am. 1957;47:545–556. - PubMed
- Birch D.G, Sandberg M.A, Berson E.L. The Stiles–Crawford effect in retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1982;22:157–164. - PubMed
- Blank K, Provine R.R, Enoch J.M. Shift in the peak of the photopic Stiles–Crawford function with marked accommodation. Vision Res. 1975;15:499–507. doi:10.1016/0042-6989(75)90027-9 - DOI - PubMed
- Borwein B. The retinal receptor: a description. In: Enoch J.M, Tobey F.L, editors. Vertebrate photoreceptor optics. Springer; Berlin, Germany: 1981. pp. 11–81.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources