Mechanoenzymatics of titin kinase - PubMed (original) (raw)
Mechanoenzymatics of titin kinase
Elias M Puchner et al. Proc Natl Acad Sci U S A. 2008.
Erratum in
- Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):21045
Abstract
Biological responses to mechanical stress require strain-sensing molecules, whose mechanically induced conformational changes are relayed to signaling cascades mediating changes in cell and tissue properties. In vertebrate muscle, the giant elastic protein titin is involved in strain sensing via its C-terminal kinase domain (TK) at the sarcomeric M-band and contributes to the adaptation of muscle in response to changes in mechanical strain. TK is regulated in a unique dual autoinhibition mechanism by a C-terminal regulatory tail, blocking the ATP binding site, and tyrosine autoinhibition of the catalytic base. For access to the ATP binding site and phosphorylation of the autoinhibitory tyrosine, the C-terminal autoinhibitory tail needs to be removed. Here, we use AFM-based single-molecule force spectroscopy, molecular dynamics simulations, and enzymatics to study the conformational changes during strain-induced activation of human TK. We show that mechanical strain activates ATP binding before unfolding of the structural titin domains, and that TK can thus act as a biological force sensor. Furthermore, we identify the steps in which the autoinhibition of TK is mechanically relieved at low forces, leading to binding of the cosubstrate ATP and priming the enzyme for subsequent autophosphorylation and substrate turnover.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
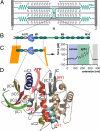
Sarcomeric location and structure of the investigated TK protein construct. (A) Schematic diagram of the sarcomere showing the transverse Z- and M-bands and actin and myosin filaments, linked by the elastic titin filament. M-bands cross-link myosin filaments by a complex of titin (green), obscurin, and myomesin (15). (B) Domain structure of M-band titin showing the array of structural Ig (green), Fn3 domains (white), and unique sequences (green lines) surrounding titin kinase domain (TK) (blue). (C) The titin construct A168-M2 contains the kinase domain surrounded by Fn3 and Ig domains. ATP binding requires relief of the C-terminal autoinhibitory tail (blue) from the active site, which can be achieved by external force. In mechanical single-molecule experiments, A168-M2 is pulled off the gold support (yellow line) by a cantilever, resulting in a unique force spectrum when the protein is stretched and domains sequentially unfold. Analysis of the unfolding force spectrum (see
SI Text
) identifies the peaks shaded in blue as kinase unfolding peaks; the five unfolding peaks shaded in green correspond to sequential Ig and Fn domain unfolding. (D) Kinase domain structure, with the ATP binding site highlighted by the pink arrow and individual secondary structure elements color-coded. Numbering is from N to C terminus, where C1 to C10 refer to catalytic core structures, and R1 to R3 (in red) refer to the regulatory tail (5). The N and C termini are marked.
Fig. 2.
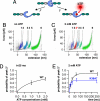
Unfolding profile of TK and kinetics of mechanically induced ATP binding. (A) External force can open the ATP binding site of TK by unfolding of the autoinhibitory domain (blue ball). (B) Superimposed traces of 66 single-molecule unfolding events in TK show a fixed sequence of local unfolding events, numbered 1–5. (C) Mechanically induced ATP binding leads to a distinctly altered force profile with the appearance of an extra force peak, 2*, absent in unfolding events in the absence of ATP (44 traces). (D) ATP binding probability (i.e., the occurrence of peak 2*) depends on ATP concentrations, giving access to virtual direct kinetics though the system is not in equilibrium (ATP peaks detected at pulling rates of 720 nm/s, or after 25 ms). (E) Probability of ATP binding depends on pulling rates, decreasing at faster speeds because of reduced opening time of the ATP binding site. ATP binding is strongly reduced by mutation of lysine-36 to alanine (K36A).
Fig. 3.
Molecular dynamics (MD) simulations of the force-induced unfolding of titin kinase (TK). (A) Representative unfolding intermediates with the unfolding secondary structure elements colored according to the scheme in Fig. 1_D_; the β-strands unfold pairwise, and colors refer to the respective N-terminal strand of each pair. (B) Unfolding forces of truncated TK with ATP (Top), without ATP (Middle), and of the complete TK (Bottom). For the complete TK, two independent 90-ns simulations were carried out (solid and dashed lines). Starting from a partially unfolded structure at ≈19 nm, five 26-ns trajectories (thin gray lines) were averaged for both sets of simulations (thick lines in Top and Middle). Color-shaded areas indicate main unfolding events, which correspond to the colors used in A and in Fig. 1_D_. An additional force peak in the presence of ATP is predicted (plus sign and pink-shaded area in Top). This force peak (Inset) is higher for bound ATP (270 pN) than for an empty binding pocket (188 pN). Because of the necessarily much faster pulling rates of 0.8 m/s used for the simulations, larger unfolding forces are seen, which can be related to the experimental loading rates (11). (C) In the force–probe MD simulations, harmonic springs were attached to the protein and retracted with constant velocity (lower schematic, ATP shown as red spheres). (C Insets) Representative structures shortly before (Left) and after (Right) the ATP force peak. ATP and the two key residues methionine-34 and lysine-36 are shown in ball-and-stick representation, and the rupture of molecular interactions is indicated by dotted lines.
Fig. 4.
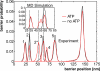
Contour length histograms obtained from single-molecule force spectroscopy experiments (transformation with QM-WLC and P = 0.8 nm) and from MD simulations (Inset). The folded kinase construct has a length of 25 nm. The peak positions with (red) and without (black) ATP are similar in both histograms (dashed lines), except for one additional peak in the presence of ATP (red peak at ≈51.6 nm). The experimentally determined contour length increments are, in the absence of ATP, 9.1, 28.6, 7.3, 18.0, 57.9 nm; and, in the presence of ATP, 9.1, 19.4, 10.1, 7.5, 16.4, 58.3 nm—with an estimated error of ±2%. The position of the initial peak (24 nm) reflects the mean length of the TK construct with completely folded domains.
Fig. 5.
Autophosphorylation of TK on tyrosine. Incubation of the highly purified TK-kin3 enzyme in the absence (−) and presence (+) of ATP and Mn2+ ions leads to tyrosine phosphorylation detected by Western blot, using the phosphotyrosine antibody 4G10. The autoinhibited kinase construct A168-M2 (WT) shows no appreciable phosphotyrosine incorporation under any tested condition. Lower blot: loading control, detection with anti-titin kinase antibody (α-TK).
Similar articles
- Single-molecule force spectroscopy reveals a stepwise unfolding of Caenorhabditis elegans giant protein kinase domains.
Greene DN, Garcia T, Sutton RB, Gernert KM, Benian GM, Oberhauser AF. Greene DN, et al. Biophys J. 2008 Aug;95(3):1360-70. doi: 10.1529/biophysj.108.130237. Epub 2008 Apr 4. Biophys J. 2008. PMID: 18390597 Free PMC article. - Mechanically induced titin kinase activation studied by force-probe molecular dynamics simulations.
Gräter F, Shen J, Jiang H, Gautel M, Grubmüller H. Gräter F, et al. Biophys J. 2005 Feb;88(2):790-804. doi: 10.1529/biophysj.104.052423. Epub 2004 Nov 5. Biophys J. 2005. PMID: 15531631 Free PMC article. - A conditional gating mechanism assures the integrity of the molecular force-sensor titin kinase.
Stahl SW, Puchner EM, Alexandrovich A, Gautel M, Gaub HE. Stahl SW, et al. Biophys J. 2011 Oct 19;101(8):1978-86. doi: 10.1016/j.bpj.2011.09.027. Biophys J. 2011. PMID: 22004752 Free PMC article. - Pulling single molecules of titin by AFM--recent advances and physiological implications.
Linke WA, Grützner A. Linke WA, et al. Pflugers Arch. 2008 Apr;456(1):101-15. doi: 10.1007/s00424-007-0389-x. Epub 2007 Dec 6. Pflugers Arch. 2008. PMID: 18058125 Review. - Cytoskeletal protein kinases: titin and its relations in mechanosensing.
Gautel M. Gautel M. Pflugers Arch. 2011 Jul;462(1):119-34. doi: 10.1007/s00424-011-0946-1. Epub 2011 Mar 18. Pflugers Arch. 2011. PMID: 21416260 Free PMC article. Review.
Cited by
- Single-molecule dissection of the high-affinity cohesin-dockerin complex.
Stahl SW, Nash MA, Fried DB, Slutzki M, Barak Y, Bayer EA, Gaub HE. Stahl SW, et al. Proc Natl Acad Sci U S A. 2012 Dec 11;109(50):20431-6. doi: 10.1073/pnas.1211929109. Epub 2012 Nov 27. Proc Natl Acad Sci U S A. 2012. PMID: 23188794 Free PMC article. - Principles and regulation of mechanosensing.
Sala S, Caillier A, Oakes PW. Sala S, et al. J Cell Sci. 2024 Sep 15;137(18):jcs261338. doi: 10.1242/jcs.261338. Epub 2024 Sep 19. J Cell Sci. 2024. PMID: 39297391 Review. - At the Start of the Sarcomere: A Previously Unrecognized Role for Myosin Chaperones and Associated Proteins during Early Myofibrillogenesis.
Myhre JL, Pilgrim DB. Myhre JL, et al. Biochem Res Int. 2012;2012:712315. doi: 10.1155/2012/712315. Epub 2012 Jan 30. Biochem Res Int. 2012. PMID: 22400118 Free PMC article. - Protein conformational switches: from nature to design.
Ha JH, Loh SN. Ha JH, et al. Chemistry. 2012 Jun 25;18(26):7984-99. doi: 10.1002/chem.201200348. Epub 2012 Jun 11. Chemistry. 2012. PMID: 22688954 Free PMC article. Review. - Nano-Precision Tweezers for Mechanosensitive Proteins and Beyond.
Yang T, Park C, Rah SH, Shon MJ. Yang T, et al. Mol Cells. 2022 Jan 31;45(1):16-25. doi: 10.14348/molcells.2022.2026. Mol Cells. 2022. PMID: 35114644 Free PMC article. Review.
References
- Tskhovrebova L, Trinick J. Titin: Properties and family relationships. Nat Rev Mol Cell Biol. 2003;4:679–689. - PubMed
- Mayans O, et al. Structural basis of the activation of the titin kinase domain during myofibrillogenesis. Nature. 1998;395:863–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous