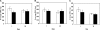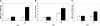Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage - PubMed (original) (raw)
Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage
Joel K Wise et al. Tissue Eng Part A. 2009 Apr.
Abstract
Cell differentiation, adhesion, and orientation are known to influence the functionality of both natural and engineered tissues, such as articular cartilage. Several attempts have been devised to regulate these important cellular behaviors, including application of inexpensive but efficient electrospinning that can produce patterned extracellular matrix (ECM) features. Electrospun and oriented polycaprolactone (PCL) scaffolds (500 or 3000 nm fiber diameter) were created, and human mesenchymal stem cells (hMSCs) were cultured on these scaffolds. Cell viability, morphology, and orientation on the fibrous scaffolds were quantitatively determined as a function of time. While the fiber-guided initial cell orientation was maintained even after 5 weeks, cells cultured in the chondrogenic media proliferated and differentiated into the chondrogenic lineage, suggesting that cell orientation is controlled by the physical cues and minimally influenced by the soluble factors. Based on assessment by the chondrogenic markers, use of the nanofibrous scaffold (500 nm) appears to enhance the chondrogenic differentiation. These findings indicate that hMSCs seeded on a controllable PCL scaffold may lead to an alternate methodology to mimic the cell and ECM organization that is found, for example, in the superficial zone of articular cartilage.
Figures
FIG. 1.
SEM images of oriented electrospun PCL fibrous scaffolds. The average fiber diameter was estimated using an image processor: (A) 500 nm; (B) 3000 nm. (C) shows nonelectrospun porous PCL film.
FIG. 2.
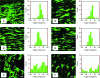
Quantitative assessment of fiber-guided cell orientation. Viable hMSCs (green) cultured in normal growth media for 4 days on electrospun oriented PCL fibrous scaffolds of average fiber diameters of 500 nm (A), and 3000 nm (B), as well as on nonelectrospun PCL film (C). Images of viable hMSCs cultured in the chondrogenic differentiation media for 4 days on electrospun oriented PCL scaffolds of average fiber diameters of 500 nm (D) and 3000 nm (E), and on nonelectrospun PCL film (F). Adjacent to each cell image are histograms representing the corresponding distributions of cell orientation angles with respect to the horizontal axis. Approximately 80 cells (except those on the PCL film) were used to construct the histograms. Scale bar denotes 75 μm. Color images available online at
.
FIG. 3.
Time-dependent changes of the SD of cell angle measurements. Cultured either in normal growth media (A) or chondrogenic differentiation media (B), the cell angle measurements were performed at day 4, 7, 21, and 35. SDs of the measurements were calculated for cells aligned on the nanofibrous (black bars), microfibrous (open bars) scaffolds, and on the non-electrospun PCL film (hatched bars). The fiber-guided initial cell alignment was essentially maintained for at least 35 days.
FIG. 4.
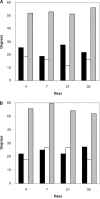
Total DNA amount measured at days 1 and 35. hMSCs cultured in growth media (open bars) or chondrogenic differentiation media (black bars) on electrospun oriented PCL fibrous scaffolds of average fiber diameters of 500 nm (A) and 3000 nm (B), as well as on nonelectrospun PCL film (C). Results represent the mean ± SEM of three independent experiments.
FIG. 5.
Normalized sGAG content measurement at days 1 and 35. hMSCs cultured either in growth media (open bars) or in chondrogenic differentiation media (black bars) on electrospun oriented PCL fibrous scaffolds having average fiber diameters of 500 nm (A) and 3000 nm (B), as well as on nonelectrospun PCL film (C). Results represent the mean ± SEM of three independent experiments.
FIG. 6.
PCR analysis of gene expressions for chondrogenic markers. The collagen type II gene (black bars) and aggregan gene (hatched bars) expression was measured from cells cultured in the chondrogenic differentiation media for 35 days on the nano- or microfibrous scaffolds and PCL film. GAPDH was used as a housekeeping control. The comparative CT method was used to show the fold-increases of the PCR results from samples cultured for 35 days in the chondrogenic differentiation media to those at day 1.
FIG. 7.
Fluorescent images of reorganized microfilaments. Following 4 days of cell culture on a nanofibrous scaffold, microfilaments were visualized using rhodamine-phalloidine. Cells seeded on a typical culture dish demonstrate a spread cellular morphology and formation of thick bundle of stress fibers (A). In contrast, cells seeded on the nanofibrous PCL scaffold exhibit an elongated cellular morphology, as expected. Moreover, stress microfilament bundles were much less pronounced, but instead regions of concentrated actins were clearly seen (B). The actin-containing tether-like adhesions between cells and fibers (circled region) were observed only when the cells were cultured on the nanofibrous scaffold, and therefore appear to be unique for cell adhesion to nanofibers. Because the PCL fibers were imaged using reflection mode, distinction between fluorescently labeled microfilaments and nanofibers may not be obvious in this composite image. An arrow was drawn in the image to indicate the location of a PCL nanofiber. A magnified image (C) better illustrates the cell attachment to a nanofiber through a tether-like adhesion mechanism that utilizes F-actins.
Similar articles
- Effects of Fiber Alignment and Coculture with Endothelial Cells on Osteogenic Differentiation of Mesenchymal Stromal Cells.
Yao T, Chen H, Baker MB, Moroni L. Yao T, et al. Tissue Eng Part C Methods. 2020 Jan;26(1):11-22. doi: 10.1089/ten.TEC.2019.0232. Epub 2019 Dec 27. Tissue Eng Part C Methods. 2020. PMID: 31774033 - Suppressing mesenchymal stem cell hypertrophy and endochondral ossification in 3D cartilage regeneration with nanofibrous poly(l-lactic acid) scaffold and matrilin-3.
Liu Q, Wang J, Chen Y, Zhang Z, Saunders L, Schipani E, Chen Q, Ma PX. Liu Q, et al. Acta Biomater. 2018 Aug;76:29-38. doi: 10.1016/j.actbio.2018.06.027. Epub 2018 Jun 22. Acta Biomater. 2018. PMID: 29940371 Free PMC article. - Biomimetic poly(glycerol sebacate)/polycaprolactone blend scaffolds for cartilage tissue engineering.
Liu Y, Tian K, Hao J, Yang T, Geng X, Zhang W. Liu Y, et al. J Mater Sci Mater Med. 2019 Apr 29;30(5):53. doi: 10.1007/s10856-019-6257-3. J Mater Sci Mater Med. 2019. PMID: 31037512 - Differentiation of mesenchymal stem cells for cartilage tissue engineering: Individual and synergetic effects of three-dimensional environment and mechanical loading.
Panadero JA, Lanceros-Mendez S, Ribelles JL. Panadero JA, et al. Acta Biomater. 2016 Mar;33:1-12. doi: 10.1016/j.actbio.2016.01.037. Epub 2016 Jan 27. Acta Biomater. 2016. PMID: 26826532 Review. - Recent Advances in "Functional Engineering of Articular Cartilage Zones by Polymeric Biomaterials Mediated with Physical, Mechanical, and Biological/Chemical Cues".
Dehghan-Baniani D, Mehrjou B, Chu PK, Lee WYW, Wu H. Dehghan-Baniani D, et al. Adv Healthc Mater. 2023 Apr;12(10):e2202581. doi: 10.1002/adhm.202202581. Epub 2023 Jan 18. Adv Healthc Mater. 2023. PMID: 36571465 Review.
Cited by
- Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues.
Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. Kim IL, et al. Biomaterials. 2013 Jul;34(22):5571-80. doi: 10.1016/j.biomaterials.2013.04.004. Epub 2013 Apr 24. Biomaterials. 2013. PMID: 23623322 Free PMC article. - The influence of tissue microenvironment on stem cell-based cartilage repair.
Jayasuriya CT, Chen Y, Liu W, Chen Q. Jayasuriya CT, et al. Ann N Y Acad Sci. 2016 Nov;1383(1):21-33. doi: 10.1111/nyas.13170. Epub 2016 Jul 27. Ann N Y Acad Sci. 2016. PMID: 27464254 Free PMC article. Review. - Bilayer Implants: Electromechanical Assessment of Regenerated Articular Cartilage in a Sheep Model.
Schagemann JC, Rudert N, Taylor ME, Sim S, Quenneville E, Garon M, Klinger M, Buschmann MD, Mittelstaedt H. Schagemann JC, et al. Cartilage. 2016 Oct;7(4):346-60. doi: 10.1177/1947603515623992. Epub 2016 Jan 22. Cartilage. 2016. PMID: 27688843 Free PMC article. - Development of three-layer collagen scaffolds to spatially direct tissue-specific cell differentiation for enthesis repair.
Pugliese E, Sallent I, Ribeiro S, Trotier A, Korntner SH, Bayon Y, Zeugolis DI. Pugliese E, et al. Mater Today Bio. 2023 Mar 7;19:100584. doi: 10.1016/j.mtbio.2023.100584. eCollection 2023 Apr. Mater Today Bio. 2023. PMID: 36969698 Free PMC article. - Evaluation of the Usability of a Low-Cost 3D Printer in a Tissue Engineering Approach for External Ear Reconstruction.
Kuhlmann C, Blum JC, Schenck TL, Giunta RE, Wiggenhauser PS. Kuhlmann C, et al. Int J Mol Sci. 2021 Oct 28;22(21):11667. doi: 10.3390/ijms222111667. Int J Mol Sci. 2021. PMID: 34769096 Free PMC article.
References
- Hunziker E.B. Articular cartilage structure in humans and experimental animals. In: Kuettner, editor. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992. pp. 183–199.
- Temenoff J.S. Mikos A.G. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431. - PubMed
- Poole A.R. Kojima T. Yasuda T. Mwale F. Kobayashi M. Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;399 Suppl:S26. - PubMed
- Jackson D.W. Scheer M.J. Simon T.M. Cartilage substitutes: overview of basic science and treatment options. J Am Acad Orthop Surg. 2001;9:37. - PubMed
- Hunziker E.B. Quinn T.M. Haeuselmann H.-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM060741-02/GM/NIGMS NIH HHS/United States
- P40RR017447/RR/NCRR NIH HHS/United States
- R21 EB006067-02/EB/NIBIB NIH HHS/United States
- R21 EB006067-01/EB/NIBIB NIH HHS/United States
- R01 GM060741-03/GM/NIGMS NIH HHS/United States
- P40 RR017447/RR/NCRR NIH HHS/United States
- R21 EB006067/EB/NIBIB NIH HHS/United States
- R01 GM060741/GM/NIGMS NIH HHS/United States
- R01 GM060741-04/GM/NIGMS NIH HHS/United States
- EB06067/EB/NIBIB NIH HHS/United States
- R01 GM060741-05/GM/NIGMS NIH HHS/United States
- R01 GM060741-01A2/GM/NIGMS NIH HHS/United States
- GM60741/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources