Highly efficient transient gene expression and gene targeting in primate embryonic stem cells with helper-dependent adenoviral vectors - PubMed (original) (raw)
Highly efficient transient gene expression and gene targeting in primate embryonic stem cells with helper-dependent adenoviral vectors
Keiichiro Suzuki et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Human embryonic stem (hES) cells are regarded as a potentially unlimited source of cellular materials for regenerative medicine. For biological studies and clinical applications using primate ES cells, the development of a general strategy to obtain efficient gene delivery and genetic manipulation, especially gene targeting via homologous recombination (HR), would be of paramount importance. However, unlike mouse ES (mES) cells, efficient strategies for transient gene delivery and HR in hES cells have not been established. Here, we report that helper-dependent adenoviral vectors (HDAdVs) were able to transfer genes in hES and cynomolgus monkey (Macaca fasicularis) ES (cES) cells efficiently. Without losing the undifferentiated state of the ES cells, transient gene transfer efficiency was approximately 100%. Using HDAdVs with homology arms, approximately one out of 10 chromosomal integrations of the vector was via HR, whereas the rate was only approximately 1% with other gene delivery methods. Furthermore, in combination with negative selection, approximately 45% of chromosomal integrations of the vector were targeted integrations, indicating that HDAdVs would be a powerful tool for genetic manipulation in hES cells and potentially in other types of human stem cells, such as induced pluripotent stem (iPS) cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
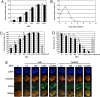
Transient gene expression in primate ES cells using HDAdVs. (A) Transient gene expression efficiencies in cES cells. CMK6 cells were infected with HDAdVenus-geo-TK, pseudotyped with Ad5 (open bars) or Ad5/35 fiber (filled bars), at various MOIs. The average of three independent experiments is shown with a standard error bar. (B) Time course of transient gene expression in cES cells. CMK6 cells were infected with HDAdVenus-geo-TK, pseudotyped with Ad5 fiber, at an MOI of 300. The average of three independent experiments is shown with a standard error bar. (C) Transient gene expression efficiencies in hES cells. KhES-1 subline 1 cells were infected with HDAdVenus-geo-TK, pseudotyped with Ad5 fiber (open bars) or Ad5/35 fiber (filled bars), at various MOIs or were transduced with pHDAdVenus-geo-TK plasmid DNA using FuGENE HD (gray bar). The average of three independent experiments is shown with a standard error bar. *, P < 0.05, t test, between Ad5 and Ad5/35 fibers. (D) Cytotoxicity after HDAdV infection of hES cells. KhES-1 cells were infected with HDAdVenus-geo-TK, pseudotyped with Ad5 (open bars) or Ad5/35 fiber (filled bars), at various MOIs or were transduced with pHDAdVenus-geo-TK plasmid DNA using FuGENE HD (gray bar). The average of three independent experiments is shown with a standard error bar. *, P < 0.05, t test, between noninfected and infected cells. (E) Expression of stem cell markers in HDAdV-infected hES cells. KhES-1 cells were infected with HDAdVenus-geo-TK, pseudotyped with Ad5 or Ad5/35 fiber, at various MOIs. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI). The merge shows costaining of Venus and POU5F1. (Scale bars, 200 μm.)
Fig. 2.
Gene targeting of the HPRT1 locus in cES cells. WT, noninfected wild-type CMK6 cells; KO, _HPRT1_-knockout cES cells. (A) Structures of the HDAdV targeting vector, the cynomolgus monkey HPRT1 locus, and the targeted locus. The probes for Southern blot analyses are shown as black bars. Venus, the Venus expression cassette driven by CS2 promoter; IRES, internal ribosomal entry site; βgeo, a fusion of the β-galactocidase and the neomycin resistant genes; pA, SV40 polyadenylation signal; S, _Sac_I sites. (B) Analysis of the _Sac_I-digested genomic structure at the cynomolgus monkey HPRT1 locus by Southern hybridization. (C) Expression of stem cell markers in the _HPRT1_-knockout cES cells. ALP, alkaline phosphatase. (Scale bars: 200 μm.) (D) Multipotency of the HPRT1_-knockout cES clone. The cells were induced to form EBs in vitro and analyzed by RT-PCR for expression of the following lineage-specific markers: GATA4 and GATA6 for endoderm; α-myosin heavy chain (α_-MHC) for mesoderm; neurogenic differentiation-1 (ND-1) and neurofilament 68 kDa (NF) for ectoderm; CDX2 for trophectoderm; NANOG for undifferentiated cES cells. HPRT1 mRNA expression disappeared in _HPRT1_-knockout cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. ES, embryonic stem; EBs, embryoid bodies; RT (−), PCR analysis without reverse transcriptase. (E) In vivo differentiation of a _HPRT1_-knockout cES clone. Hematoxylin and eosin staining of teratoma formed after injection of _HPRT1_-knockout cES cells into a SCID mouse. Tissues derived from three germ layers were found. (Scale bars, 100 μm.)
Fig. 3.
Gene targeting of the HPRT1 locus in hES cells. WT, noninfected wild-type KhES-1 cells; KO, HPRT1_-knockout hES cells. (A) Structures of the HDAdV targeting vector, the human HPRT1 locus, and the targeted locus. The probes for Southern blot analyses are shown as black bars. Arrows (P1 and P2) indicate a pair of primers for PCR analysis. HSV_tk, the herpes simplex virus thymidine kinase gene expression cassette driven by MC1 promoter; PGK-neo, the neomycin-resistant gene expression cassette driven by PGK promoter; Venus, the Venus expression cassette driven by CS2 promoter; H, _Hpa_I sites; Sb, Sbf I sites. PCR analysis (B) and Southern blot analysis (C) at the HPRT1 locus. Because KhES-1 is a female cell line with two X chromosomes, HR resulted in one band corresponding to the targeted allele and another from the residual unmodified allele. M, Kb DNA Ladder (STRATAGENE).
Similar articles
- Efficient and accurate homologous recombination in hESCs and hiPSCs using helper-dependent adenoviral vectors.
Aizawa E, Hirabayashi Y, Iwanaga Y, Suzuki K, Sakurai K, Shimoji M, Aiba K, Wada T, Tooi N, Kawase E, Suemori H, Nakatsuji N, Mitani K. Aizawa E, et al. Mol Ther. 2012 Feb;20(2):424-31. doi: 10.1038/mt.2011.266. Epub 2011 Dec 6. Mol Ther. 2012. PMID: 22146343 Free PMC article. - Correction of chromosomal mutation and random integration in embryonic stem cells with helper-dependent adenoviral vectors.
Ohbayashi F, Balamotis MA, Kishimoto A, Aizawa E, Diaz A, Hasty P, Graham FL, Caskey CT, Mitani K. Ohbayashi F, et al. Proc Natl Acad Sci U S A. 2005 Sep 20;102(38):13628-33. doi: 10.1073/pnas.0506598102. Epub 2005 Sep 7. Proc Natl Acad Sci U S A. 2005. PMID: 16174752 Free PMC article. - Gene targeting in human-induced pluripotent stem cells with adenoviral vectors.
Mitani K. Mitani K. Methods Mol Biol. 2014;1114:163-7. doi: 10.1007/978-1-62703-761-7_10. Methods Mol Biol. 2014. PMID: 24557902 - [Optimization of adenovirus vectors for transduction in embryonic stem cells and induced pluripotent stem cells].
Tashiro K. Tashiro K. Yakugaku Zasshi. 2011;131(9):1333-8. doi: 10.1248/yakushi.131.1333. Yakugaku Zasshi. 2011. PMID: 21881308 Review. Japanese. - Adenovirus vector-mediated gene transfer into stem cells.
Kawabata K, Sakurai F, Koizumi N, Hayakawa T, Mizuguchi H. Kawabata K, et al. Mol Pharm. 2006 Mar-Apr;3(2):95-103. doi: 10.1021/mp0500925. Mol Pharm. 2006. PMID: 16579638 Review.
Cited by
- Progress and prospects in stem cell therapy.
Xu XL, Yi F, Pan HZ, Duan SL, Ding ZC, Yuan GH, Qu J, Zhang HC, Liu GH. Xu XL, et al. Acta Pharmacol Sin. 2013 Jun;34(6):741-6. doi: 10.1038/aps.2013.77. Acta Pharmacol Sin. 2013. PMID: 23736002 Free PMC article. Review. - Targeted Integration and High-Level Transgene Expression in AAVS1 Transgenic Mice after In Vivo HSC Transduction with HDAd5/35++ Vectors.
Li C, Mishra AS, Gil S, Wang M, Georgakopoulou A, Papayannopoulou T, Hawkins RD, Lieber A. Li C, et al. Mol Ther. 2019 Dec 4;27(12):2195-2212. doi: 10.1016/j.ymthe.2019.08.006. Epub 2019 Aug 19. Mol Ther. 2019. PMID: 31494053 Free PMC article. - Efficient integration of transgenes into a defined locus in human embryonic stem cells.
Sakurai K, Shimoji M, Tahimic CG, Aiba K, Kawase E, Hasegawa K, Amagai Y, Suemori H, Nakatsuji N. Sakurai K, et al. Nucleic Acids Res. 2010 Apr;38(7):e96. doi: 10.1093/nar/gkp1234. Epub 2010 Jan 13. Nucleic Acids Res. 2010. PMID: 20071742 Free PMC article. - Dual usage of a stage-specific fluorescent reporter system based on a helper-dependent adenoviral vector to visualize osteogenic differentiation.
Sone T, Shin M, Ouchi T, Sasanuma H, Miyamoto A, Ohte S, Tsukamoto S, Nakanishi M, Okano H, Katagiri T, Mitani K. Sone T, et al. Sci Rep. 2019 Jul 4;9(1):9705. doi: 10.1038/s41598-019-46105-y. Sci Rep. 2019. PMID: 31273280 Free PMC article. - An evolved adeno-associated viral variant enhances gene delivery and gene targeting in neural stem cells.
Jang JH, Koerber JT, Kim JS, Asuri P, Vazin T, Bartel M, Keung A, Kwon I, Park KI, Schaffer DV. Jang JH, et al. Mol Ther. 2011 Apr;19(4):667-75. doi: 10.1038/mt.2010.287. Epub 2011 Jan 11. Mol Ther. 2011. PMID: 21224831 Free PMC article.
References
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. - PubMed
- Pera MF, Roach S, Elliss CJ. Comparative biology of mouse and human embryonal carcinoma. Cancer Surv. 1990;9:243–262. - PubMed
- Ginis I, et al. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269:360–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous