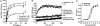Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics - PubMed (original) (raw)
Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics
Bernard Masri et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Since the unexpected discovery of the antipsychotic activity of chlorpromazine, a variety of therapeutic agents have been developed for the treatment of schizophrenia. Despite differences in their activities at various neurotransmitter systems, all clinically effective antipsychotics share the ability to interact with D2 class dopamine receptors (D2R). D2R mediate their physiological effects via both G protein-dependent and independent (beta-arrestin 2-dependent) signaling, but the role of these D2R-mediated signaling events in the actions of antipsychotics remains unclear. We demonstrate here that while different classes of antipsychotics have complex pharmacological profiles at G protein-dependent D2R long isoform (D2(L)R) signaling, they share the common property of antagonizing dopamine-mediated interaction of D2(L)R with beta-arrestin 2. Using two cellular assays based on a bioluminescence resonance energy transfer (BRET) approach, we demonstrate that a series of antipsychotics including haloperidol, clozapine, aripiprazole, chlorpromazine, quetiapine, olanzapine, risperidone, and ziprasidone all potently antagonize the beta-arrestin 2 recruitment to D2(L)R induced by quinpirole. However, these antipsychotics have various effects on D2(L)R mediated G(i/o) protein activation ranging from inverse to partial agonists and antagonists with highly variable efficacies and potencies at quinpirole-induced cAMP inhibition. These results suggest that the different classes of clinically effective antipsychotics share a common molecular mechanism involving inhibition of D2(L)R/beta-arrestin 2 mediated signaling. Thus, selective targeting of D2(L)R/beta-arrestin 2 interaction and related signaling pathways may provide new opportunities for antipsychotic development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
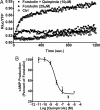
BRET measurements of cAMP levels in living cells to monitor dopamine D2L receptor activation. (A and B) Variations of cAMP levels in HEK293 cells stably expressing the EPAC biosensor and D2LR. (A) Kinetics of cAMP variations after treatment of the cells at time 0 with forskolin (25 μM) in the absence (▵) or presence (■) of quinpirole 1 μM as indicated and the emission ratios (_R_luc/YFP) were measured. Cells incubated in PBS (○) were used as a negative control. (B) Dose-response curve of quinpirole for inhibiting forskolin-stimulated cAMP accumulation in HEK cells. cAMP production was normalized to the percentage of forskolin-stimulated cAMP accumulation (set at 100%).
Fig. 2.
BRET measurements of β-arrestin 2 recruitment to dopamine D2L receptor in living cells. (A) BRET titration curve for the β-arrestin 2 recruitment to D2LR. BRET was measured in cells expressing a fixed amount of _R_luc-tagged D2LR and increasing amounts of YFP-tagged β-arrestin 2, treated (with dopamine 1 μM ▾ or quinpirole 1 μM (■) or not (♦). Specificity of the BRET signal between D2LR and the β-arrestin 2 was controlled by measuring BRET between _R_luc-tagged D2LR and YFP alone, treated or not with dopamine and quinpirole (see
Fig. S2
). (B) Kinetic of β-arrestin 2 recruitment to D2LR after addition, at time 0, of quinpirole 1 μM (■) or PBS (○). As a negative control, similar experiments were carried out on cells transiently expressing only _R_luc-tagged D2LR. (C) Dose-response curve of quinpirole for β-arrestin 2 recruitment to D2LR. Cells coexpressing _R_luc-tagged D2LR and YFP-tagged β-arrestin 2 were stimulated with dopamine (○) or quinpirole (■). Results are expressed in Net BRET as described in the Methods section. Data represent the mean ± SEM of 3 to 7 independent experiments each performed in duplicate.
Fig. 3.
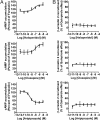
Intrinsic activity of haloperidol, clozapine, and aripiprazole on Gi/o activation and D2LR -mediated β-arrestin 2 recruitment. (A) Dose–response of haloperidol, clozapine, and aripiprazole on adenylylcyclase inhibition through D2R. As described in Methods section, HEK 293 cells stably expressing EPAC biosensor and D2R were stimulated with haloperidol (Upper Left), clozapine (Middle Left) or aripiprazole (Bottom Left) in presence of forskolin (25 μM). cAMP production was normalized to the percentage of forskolin-stimulated cAMP accumulation (set at 100%). (B) BRET measured in cells coexpressing _R_luc-tagged D2LR and YFP-tagged β-arrestin 2 and stimulated with haloperidol (Upper Right), clozapine and aripiprazole (Middle and Bottom Right, respectively). Data are expressed as the percentage of quinpirole maximum effect (1 μM) and represent the mean ± SEM of 3–5 independent experiments each performed in duplicate.
Fig. 4.
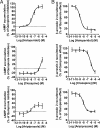
Antagonist activity of haloperidol, clozapine, and aripiprazole on Gi/o activation and β-arrestin 2 recruitment induced by quinpirole. (A) Dose-response curve for haloperidol, clozapine and aripiprazole (Upper, Middle, and Bottom Left) for inhibiting adenylylcyclase inhibition induced by quinpirole. HEK 293 cells stably coexpressing EPAC biosensor and D2LR were treated with the different antipsychotics and quinpirole (1 μM) in the presence of forskolin (25 μM). (B) Dose-response curve for haloperidol (Upper Right), clozapine and aripiprazole (M_iddle and Bottom Right_, respectively) for inhibiting β-arrestin 2 recruitment induced by 1 μM of quinpirole. BRET was measured in cells coexpressing _R_luc-tagged D2LR and YFP-tagged β-arrestin 2. Data represent the mean ± SEM of 3–5 independent experiments each performed in duplicate.
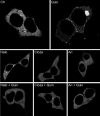
Fig. 5.
β-arrestin 2-YFP translocation to D2LR. Fluorescence microscopy was used to visualize β-arrestin 2-YFP recruitment to the human D2R long form in cells incubated for 30 min with: no drug (Ctr), haloperidol (10 μM) (Halo), clozapine (10 μM) (Cloza), aripiprazole (10 μM) (Ari), quinpirole (10 μM) (Quin). For antagonistic activity (Bottom), cells were pretreated with the different antipsychotics for 30 min and then stimulated for 30 min with quinpirole (10 μM). Representative pictures of each condition are shown (n = 3).
Similar articles
- Recruitment of beta-arrestin2 to the dopamine D2 receptor: insights into anti-psychotic and anti-parkinsonian drug receptor signaling.
Klewe IV, Nielsen SM, Tarpø L, Urizar E, Dipace C, Javitch JA, Gether U, Egebjerg J, Christensen KV. Klewe IV, et al. Neuropharmacology. 2008 Jun;54(8):1215-22. doi: 10.1016/j.neuropharm.2008.03.015. Epub 2008 Apr 8. Neuropharmacology. 2008. PMID: 18455202 Free PMC article. - Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy.
Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M, Peterson S, Yadav PN, Huang XP, Feng B, Jensen NH, Che X, Bai X, Frye SV, Wetsel WC, Caron MG, Javitch JA, Roth BL, Jin J. Allen JA, et al. Proc Natl Acad Sci U S A. 2011 Nov 8;108(45):18488-93. doi: 10.1073/pnas.1104807108. Epub 2011 Oct 24. Proc Natl Acad Sci U S A. 2011. PMID: 22025698 Free PMC article. - Discovery and characterization of a G protein-biased agonist that inhibits β-arrestin recruitment to the D2 dopamine receptor.
Free RB, Chun LS, Moritz AE, Miller BN, Doyle TB, Conroy JL, Padron A, Meade JA, Xiao J, Hu X, Dulcey AE, Han Y, Duan L, Titus S, Bryant-Genevier M, Barnaeva E, Ferrer M, Javitch JA, Beuming T, Shi L, Southall NT, Marugan JJ, Sibley DR. Free RB, et al. Mol Pharmacol. 2014 Jul;86(1):96-105. doi: 10.1124/mol.113.090563. Epub 2014 Apr 22. Mol Pharmacol. 2014. PMID: 24755247 Free PMC article. - New Concepts in Dopamine D2 Receptor Biased Signaling and Implications for Schizophrenia Therapy.
Urs NM, Peterson SM, Caron MG. Urs NM, et al. Biol Psychiatry. 2017 Jan 1;81(1):78-85. doi: 10.1016/j.biopsych.2016.10.011. Epub 2016 Oct 19. Biol Psychiatry. 2017. PMID: 27832841 Free PMC article. Review. - Atypical antipsychotics: mechanism of action.
Seeman P. Seeman P. Can J Psychiatry. 2002 Feb;47(1):27-38. Can J Psychiatry. 2002. PMID: 11873706 Review.
Cited by
- Review of serum prolactin levels as an antipsychotic-response biomarker.
Gault JM, Nussbaum AM. Gault JM, et al. Open Access J Transl Med Res. 2018;2(3):84-91. doi: 10.15406/oajtmr.2018.02.00043. Epub 2018 May 4. Open Access J Transl Med Res. 2018. PMID: 34079927 Free PMC article. - Unique Effects of Acute Aripiprazole Treatment on the Dopamine D2 Receptor Downstream cAMP-PKA and Akt-GSK3β Signalling Pathways in Rats.
Pan B, Chen J, Lian J, Huang XF, Deng C. Pan B, et al. PLoS One. 2015 Jul 10;10(7):e0132722. doi: 10.1371/journal.pone.0132722. eCollection 2015. PLoS One. 2015. PMID: 26162083 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - Viagra for your synapses: Enhancement of hippocampal long-term potentiation by activation of beta-adrenergic receptors.
O'Dell TJ, Connor SA, Gelinas JN, Nguyen PV. O'Dell TJ, et al. Cell Signal. 2010 May;22(5):728-36. doi: 10.1016/j.cellsig.2009.12.004. Epub 2009 Dec 31. Cell Signal. 2010. PMID: 20043991 Free PMC article. Review. - GSK-3β activity and hyperdopamine-dependent behaviors.
Li YC, Gao WJ. Li YC, et al. Neurosci Biobehav Rev. 2011 Jan;35(3):645-54. doi: 10.1016/j.neubiorev.2010.08.001. Epub 2010 Aug 18. Neurosci Biobehav Rev. 2011. PMID: 20727368 Free PMC article. Review.
References
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. - PubMed
- Haro JM, Salvador-Carulla L. The SOHO (Schizophrenia Outpatient Health Outcome) study: Implications for the treatment of schizophrenia. CNS Drugs. 2006;20:293–301. - PubMed
- Kroeze WK, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. - PubMed
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MH-82441/MH/NIMH NIH HHS/United States
- U19 MH082441/MH/NIMH NIH HHS/United States
- R01 NS019576/NS/NINDS NIH HHS/United States
- NS-19576/NS/NINDS NIH HHS/United States
- R37 MH073853/MH/NIMH NIH HHS/United States
- R01 MH073853/MH/NIMH NIH HHS/United States
- MH-73853/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases