Expression of liver X receptor beta is essential for formation of superficial cortical layers and migration of later-born neurons - PubMed (original) (raw)
Expression of liver X receptor beta is essential for formation of superficial cortical layers and migration of later-born neurons
Xiaotang Fan et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Liver X receptor (LXR) beta regulates cholesterol levels in the brain and is essential for maintenance of motor neurons in the spinal cord and dopaminergic neurons in the substantia nigra. Here, we have examined the expression pattern of LXRbeta protein in the cerebral cortex and looked for defects in cortical development in LXRbeta knockout (LXRbeta(-/-)) mice. LXRbeta protein was widely expressed in the mouse brain at later embryonic stages, and the expression pattern in the cerebral cortex was developmentally regulated. In normal postnatal mice, LXRbeta was localized mainly in the upper layers of the cerebral cortex. In LXRbeta(-/-) mice layers II and III were thinner with fewer neurons. Layer I was slightly thicker, whereas layers IV-VI were essentially normal. Consistent with this finding, Brn2 and NeuN expression were decreased in the upper layers in the LXRbeta(-/-) neonatal cortex. The number of S-phase progenitor cells in the cortex between embryonic day (E) 12.5 to E16.5, was similar in WT and LXRbeta(-/-) littermates but BrdU birth dating revealed that late-generated neurons labeled by BrdU injections administered at E14.5 or E16.5, and destined to cortical layers II/III, were disorganized and failed to migrate. The defect in migration appears to be caused by a reduction in the number of vertical processes emanating from the radial glia. These processes are the architectural guides for later-born migrating neurons. Taken together, these findings suggest that LXRbeta expression in the cerebral cortex is involved in cortex lamination and is essential for the migration of late-generated neocortical neurons.
Conflict of interest statement
Conflict of interest statement: J.-Å.G. is a shareholder, grant receiver, and consultant of KaroBio AB.
Figures
Fig. 1.
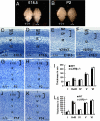
LXRβ expression in the cerebral cortex. (A) In the E14.5 brain, only a few LXRβ-positive cells are localized in the CP. (B) At E16.5, LXRβ expression in brain increased significantly; most of LXRβ-positive neurons occupy the CP, with a few positive cells in MZ and SP. (C) By E17.5, strong LXRβ expression is seen mainly in CP, and some positive cells reside in MZ and SP. (D) At P0, LXRβ is localized mainly in the MZ and upper layers of the CP, a few positive cells are scattered in deep layers. (E–H) At P9 (E and F) and P14 (G and H) strongly LXRβ-positive neurons are localized mainly in the II/III layers, with some positive cells in the layer IV, and fewer stained cells in layers V and VI. (F and H) Higher-power views of the boxed areas in E and G. (Scale bars: A, B, and D, 50 μm; C, F, and H, 100 μm; E and G, 200 μm.)
Fig. 2.
Morphological alteration of the developing neocortex in the LXRβ−/− mice. (A and B) Comparison between WT (+/+) and LXRβ−/− brains (−/−) of E18.5 (A) and P2 (B); the hemispheres are smaller and flatter in LXRβ−/− brains (−/−). (C–H, J, and K) Coronal sections of the developing cortex stained with Nissl (thionin). (C and D) There is no visible difference in the overall appearance of the cortex between WT (C) and LXRβ−/− (D) mice at E16.5. (E and F) At E18.5, the CP is thinner in the LXRβ −/− (F) mice than in WT littermates (E). (G and H) At P2, the upper layers seem apparently thinner with fewer neurons in LXRβ−/− (H) mice compared with WT (G) mice, whereas layers IV through VI are essentially normal. (K and J) The thickness of II/III layers is significantly decreased in LXRβ−/−mice (K) compared with controls (J) at P14, whereas the thickness of layer I was lightly increased. (I and L) The thickness of cortical layers was measured at P2 (I) and P14 (L), and the results were expressed as mean ± SD. Three brains were used for each genotype. *, P < 0.05; **, P < 0.01, Student's t test. (Scale bars: C–H, 100 μm; J and K, 200 μm.)
Fig. 3.
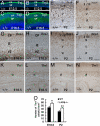
Specific reduction in upper cortical layers caused by LXRβ deficiency. (A–D) Immunolabeling of PP and SP by Tuj-1 antibody on parasagittal sections of E12.5 (A and B) and coronal sections of E16.5 (C and D) show no difference between WT and LXRβ−/− mice. (E and F) At P2, WT NeuN-stained cells are localized mostly in the II/III layers (E), whereas NeuN-positive cells are localized in layer IV in LXRβ−/− mice (F). (G–J) Specific reduction of superficial cortical layers is also shown by decreased immunostaining of Brn2 at E18.5 (G and H) and at P2 (I and J). (K–N) The relatively normal deeper cortical layer thickness is demonstrated by immunostaining with Tbr1 antibody, a marker for cortical layer VI. (O) The density of Tbr1-positive neurons in layer VI is also shown. In the LXRβ−/− mouse brain there is a significant increase in Tbr1 neuronal density (P < 0.05 at E18.5; P < 0.01 at P2 compared with WT controls, by Student's t test). (Scale bars: A–D, 100 μm; E–L, 100 μm; and M and N, 200 μm.)
Fig. 4.
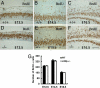
Analysis of S-phase progenitor cells by BrdU pulse labeling of WT and LXRβ−/− embryos at E12.5, E14.5, and E16.5. (A–F) A similar number of S-phase progenitor cells are in the LXRβ−/− cortex relative to the WT control. (G) The average number of BrdU-labeled cells in the SVZ was calculated at E12.5, E14.5, and E16.5 in each field under ×40 magnification (n = 3; error bar, SD). There is no difference in BrdU+ cells between WT and LXRβ−/−. (Scale bars: A–F, 100 μm.)
Fig. 5.
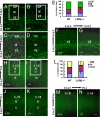
Defect in later-born neuronal migration of LXRβ−/− cortex. (A–D) Neurons labeled at E14.5 and analyzed at E18.5 accumulated in the VZ/SVZ and IZ. There are fewer labeled cells in the CP of LXRβ−/− mouse embryos (B and D) compared with WT littermates (A and C). C and D are higher-power views of the boxed areas in A and B. (E) The histogram shows the distribution of BrdU+ cells at E18.5 when labeled at E14.5 and analyzed in the different compartments of the cortex (VZ/SVZ, IZ, and CP) as a way to quantify radial migration in the different genotypes. Asterisks indicate significant differences in percentages of BrdU-positive cells in a given cortical zone in mutant and WT cortices (n = 3; **, P < 0.01). (F and G) Neurons labeled at E16.5 and analyzed at E18.5 were located mostly in the SVZ and IZ of LXRβ−/− mutant embryos (G) but in WT embryos (F) the labeled neurons had migrated to the outer layers. (H and J) When neurons were labeled at E14.5 and analyzed at P14, there were more labeled cells in layers II/III and layer IV and fewer cells in layer V and VI in the WT mouse brains. (I and K) The pattern of BrdU labeled cells is inverted in the mutant cortex, with positive cells localized mainly in the deep layers (V and VI) and with few cells in layers II/III. J and K are higher-power views of the boxed areas in H and I. (L) The histogram shows the distribution at P14 of BrdU-positive cells (labeled at E14.5) in the different compartments of the cortex (II/III, IV, and V/VI). Asterisks indicate significant differences in percentages of BrdU-positive cells in a given cortical zone in mutant and WT cortices (n = 3; **, P < 0.01). (M and N) When BrdU was administered at E16.5, and analyzed at P14, there were fewer BrdU-labeled cells in layers II/III of LXRβ−/− mice (N) compared with WT littermates (M). (Scale bars: A, B, F, G, J, and K, 100 μm; C and D, 50 μm; H, I, M, and N, 200 μm.)
Fig. 6.
Abnormalities in radial glia and normal reelin expression in LXRβ−/− mutants. (A–F) Nestin staining of E12.5, E16.5, and E18.5 cortices. (A and B) The pattern and distribution of radial glial fibers is comparable in WT (A) and LXRβ−/− (B) mice at E12.5. (C and D) At E16.5, In WT controls there are many radially distributed processes extending throughout the full thickness of the CP (C), whereas, in LXRβ−/− mice, some nestin-stained fibers fail to extend through the full thickness of the cerebral wall (D). (E and F) At E18.5, disruption of radial fibers is apparent in mutant cortices (F) compared with WT (E). (G and H) In contrast, reelin expression (green) is comparable in WT (G) and LXRβ−/− (H) mice at E18.5. Nuclei were counterstained with DAPI (blue). (Scale bars: A–F, 50 μm; G and H, 100 μm.)
Similar articles
- Liver X receptor beta and thyroid hormone receptor alpha in brain cortical layering.
Tan XJ, Fan XT, Kim HJ, Butler R, Webb P, Warner M, Gustafsson JA. Tan XJ, et al. Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12305-10. doi: 10.1073/pnas.1006162107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566868 Free PMC article. - Liver X receptor β delays transformation of radial glial cells into astrocytes during mouse cerebral cortical development.
Guo L, Xu P, Tang X, Wu Q, Xing Y, Gustafsson JA, Xu H, Fan X. Guo L, et al. Neurochem Int. 2014 May;71:8-16. doi: 10.1016/j.neuint.2014.03.009. Epub 2014 Mar 22. Neurochem Int. 2014. PMID: 24662373 - Liver X receptor beta (LXRbeta): a link between beta-sitosterol and amyotrophic lateral sclerosis-Parkinson's dementia.
Kim HJ, Fan X, Gabbi C, Yakimchuk K, Parini P, Warner M, Gustafsson JA. Kim HJ, et al. Proc Natl Acad Sci U S A. 2008 Feb 12;105(6):2094-9. doi: 10.1073/pnas.0711599105. Epub 2008 Jan 31. Proc Natl Acad Sci U S A. 2008. PMID: 18238900 Free PMC article. - Minireview: liver X receptor beta: emerging roles in physiology and diseases.
Gabbi C, Warner M, Gustafsson JA. Gabbi C, et al. Mol Endocrinol. 2009 Feb;23(2):129-36. doi: 10.1210/me.2008-0398. Epub 2008 Dec 12. Mol Endocrinol. 2009. PMID: 19074550 Free PMC article. Review. - Timing of major ontogenetic events in the visual cortex of the rhesus monkey.
Rakic P. Rakic P. UCLA Forum Med Sci. 1975;(18):3-40. doi: 10.1016/b978-0-12-139050-1.50008-2. UCLA Forum Med Sci. 1975. PMID: 812226 Review.
Cited by
- Npas3 regulates stemness maintenance of radial glial cells and neuronal migration in the developing mouse cerebral cortex.
Liu JW, Li H, Zhang Y. Liu JW, et al. Front Cell Neurosci. 2022 Oct 13;16:865681. doi: 10.3389/fncel.2022.865681. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36313621 Free PMC article. - Liver X receptors alpha and beta promote myelination and remyelination in the cerebellum.
Meffre D, Shackleford G, Hichor M, Gorgievski V, Tzavara ET, Trousson A, Ghoumari AM, Deboux C, Nait Oumesmar B, Liere P, Schumacher M, Baulieu EE, Charbonnier F, Grenier J, Massaad C. Meffre D, et al. Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7587-92. doi: 10.1073/pnas.1424951112. Epub 2015 May 28. Proc Natl Acad Sci U S A. 2015. PMID: 26023184 Free PMC article. - Allopregnanolone as regenerative therapeutic for Alzheimer's disease: translational development and clinical promise.
Irwin RW, Brinton RD. Irwin RW, et al. Prog Neurobiol. 2014 Feb;113:40-55. doi: 10.1016/j.pneurobio.2013.08.004. Epub 2013 Sep 14. Prog Neurobiol. 2014. PMID: 24044981 Free PMC article. Review. - Stromal growth and epithelial cell proliferation in ventral prostates of liver X receptor knockout mice.
Kim HJ, Andersson LC, Bouton D, Warner M, Gustafsson JA. Kim HJ, et al. Proc Natl Acad Sci U S A. 2009 Jan 13;106(2):558-63. doi: 10.1073/pnas.0811295106. Epub 2009 Jan 2. Proc Natl Acad Sci U S A. 2009. PMID: 19122149 Free PMC article. - LXR agonists: new potential therapeutic drug for neurodegenerative diseases.
Xu P, Li D, Tang X, Bao X, Huang J, Tang Y, Yang Y, Xu H, Fan X. Xu P, et al. Mol Neurobiol. 2013 Dec;48(3):715-28. doi: 10.1007/s12035-013-8461-3. Epub 2013 Apr 27. Mol Neurobiol. 2013. PMID: 23625315 Review.
References
- Alberti S, Steffensen KR, Gustafsson JA. Structural characterization of the mouse nuclear oxysterol receptor genes LXRα and LXRβ. Gene. 2000;243:93–103. - PubMed
- Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases