Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination - PubMed (original) (raw)
Trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination
David R Kaufman et al. J Immunol. 2008.
Abstract
A critical goal of vaccine development for a wide variety of pathogens is the induction of potent and durable mucosal immunity. However, it has been assumed that this goal would be difficult to achieve by systemic vaccination due to the anatomic and functional distinctness of the systemic and mucosal immune systems and the resultant compartmentalization of immune responses. In this study, we show that Ag-specific CD8(+) T lymphocytes traffic efficiently to mucosal surfaces following systemic vaccination. Intramuscular immunization with recombinant adenovirus (rAd) vector-based vaccines expressing SIV Gag resulted in potent, durable, and functional CD8(+) T lymphocyte responses at multiple mucosal effector sites in both mice and rhesus monkeys. In adoptive transfer studies in mice, vaccine-elicited systemic CD8(+) T lymphocytes exhibited phenotypic plasticity, up-regulated mucosal homing integrins and chemokine receptors, and trafficked rapidly to mucosal surfaces. Moreover, the migration of systemic CD8(+) T lymphocytes to mucosal compartments accounted for the vast majority of Ag-specific mucosal CD8(+) T lymphocytes induced by systemic vaccination. Thus, i.m. vaccination can overcome immune compartmentalization and generate robust mucosal CD8(+) T lymphocyte memory. These data demonstrate that the systemic and mucosal immune systems are highly coordinated following vaccination.
Figures
FIGURE 1
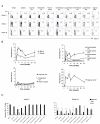
Intramuscular rAd immunization induces durable high frequency CD8+ T-lymphocyte memory in multiple mucosal compartments. C57BL/6 mice were immunized intramuscularly with 109 VP rAd5-Gag, and AL11-specific CD8+ T-lymphocyte responses were followed over a 24-week time course in multiple anatomic compartments using Db/AL11 tetramer binding assays, as shown for a representative experiment (A) and in summary for 4-6 mice/time point in (B). In (C), the memory phenotype (effector, black bars; effector memory, white bars; and central memory, gray bars) of the AL11-specific CD8+ T-lymphyocyte population was determined at weeks 2 and 24 post-immunization based on expression of CD62L and CD127. Error bars are +/− S.E.
FIGURE 2
Intramuscular rAd immunization induces high frequency functional mucosal CD8+ T-lymphocyte responses but low frequency mucosal CD4+ T-lymphocyte responses. C57BL/6 mice were immunized intramuscularly with 109 VP rAd5-Gag, and CD8+ T-lymphocyte responses to pooled Gag peptides or to the AL11 epitope peptide were monitored at week 2 post-immunization by intracellular cytokine staining for IFN-γ, as shown for a representative experiment in (A) and in summary for 6 mice/group in (B), or for IL-2 (C). C57BL/6 mice (n=6/group) were also immunized intramuscularly with 109 VP of rAd5-Gag (D) or primed with 109 VP rAd5-Gag and boosted with 109 VP rAd5HVR48-Gag (E), and CD4+ T-cell responses were assessed by intracellular cytokine staining for IFN-γ after stimulation with pooled Gag peptides at week 2 post-immunization. Error bars are +/− S.E.
FIGURE 3
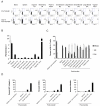
Systemic CD8+ T-lymphocytes upregulate mucosal homing markers and migrate to multiple mucosal compartments after adoptive transfer. Donor C57BL/6 mice were primed intramuscularly at week 0 with 109 VP rAd26-Gag and boosted at week 6 with 109 VP rAd5HVR48-Gag. On day 10 following the boost immunization, CD8+ T-lymphocytes were purified from splenocytes by negative immunomagnetic selection, and 2×107 purified lymphocytes were injected intravenously into naïve recipient mice (n=5/experiment). The tissue distribution of transferred AL11-specific CD8+ T-lymphocytes was determined at week 2 after adoptive transfer, as shown for a representative experiment (A) and in summary for 5 mice/group in (B). The effector and memory phenotypes (C) and the pattern of mucosal homing marker expression (D) were determined for AL11-specific CD8+ T-lymphocytes both prior to transfer and at week 2 post-transfer.
FIGURE 4
Vaccination facilitates trafficking of systemic CD8+ T-lymphocytes to mucosal surfaces. 107 purified systemic CD8+ T-lymphocytes were isolated from splenocytes of naïve CD45.1+ donors and adoptively transferred into naïve congenic CD45.2+ recipients. The ratio of transferred/native CD8+ T-lymphocytes was determined on day 12 post-transfer for the total CD8+ T-lymphocyte population at each anatomic site in the absence of immunization (A) or for the responding AL11-specific CD8+ T-lymphocyte population at each anatomic site on day 14 post-immunization with rAd5-Gag (B). In each case, the top panel demonstrates the gating strategy used for analysis, the middle panel shows a representative experiment, and the bottom panel shows the ratio of transferred/native CD8+ T-lymphocytes at each anatomic site averaged for 4 mice/group. (C) 107 purified systemic CD4+ T-lymphocytes were isolated from splenocytes of naïve CD45.1+ donors and adoptively transferred into naïve congenic CD45.2+ recipients. The ratio of transferred/native CD4+ T-lymphocytes was determined on day 14 post-transfer at each anatomic site in the absence of immunization (black bars) or following intramuscular immunization with rAd5-Gag on day 2 (white bars). Error bars are +/− S.E.
FIGURE 5
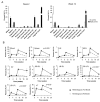
Heterologous rAd prime-boost regimens are significantly superior to homologous regimens in inducing mucosal CD8+ T-lymphocyte recall responses. (A) To compare primary and recall responses, AL11-specific CD8+ T-lymphocyte responses at week 2 and week 12 following immunization with 109 VP rAd5HVR48-Gag were evaluated in previously naïve C57BL/6 mice (white bars) or in mice primed 6 weeks earlier with 109 VP rAd26-Gag (black bars) (n=4/group at each time point). (B) C57BL/6 mice (n=4/group at each time point) were primed intramuscularly at week 0 with 109 VP rAd26-Gag and boosted at week 8 with either 109 VP rAd26-Gag (homologous vector; dashed lines) or 109 VP rAd5HVR48-Gag (heterologous vector; solid lines). AL11-specific CD8+ T-lymphocyte responses were assessed at multiple time points following the priming and boosting immunizations. Means +/− S.E. for each group are shown. Asterisks denote two-sided t-tests. ILN, inguinal lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer's patches; SB, small bowel; LB, large bowel; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes; VT, vaginal tract.
FIGURE 6
Mucosal cellular immune memory in rhesus monkeys after intramuscular rAd vaccination. Two rhesus monkeys expressing the MHC class I allele Mamu-A*01 (animals 184-03, gray bars, and 210-03, black bars) and one monkey negative for this allele (153-03, white bars) were vaccinated intramuscularly with a single injection of 1011 VP rAd5HVR48-Gag. Gag CM9-specific CD8+ T-lymphocyte responses were evaluated in systemic and mucosal compartments at weeks 4, 32, and 52 following vaccination. The memory phenotype of the responding lymphocytes was determined by CD28 and CD95 expression.
Comment in
- Comment on "trafficking of antigen-specific CD8+ T lymphocytes to mucosal surfaces following intramuscular vaccination".
Belyakov IM, Ahlers JD. Belyakov IM, et al. J Immunol. 2009 Feb 15;182(4):1779; author reply 1779-80. doi: 10.4049/jimmunol.0990002. J Immunol. 2009. PMID: 19201827 No abstract available.
Similar articles
- Durable mucosal simian immunodeficiency virus-specific effector memory T lymphocyte responses elicited by recombinant adenovirus vectors in rhesus monkeys.
Li H, Liu J, Carville A, Mansfield KG, Lynch D, Barouch DH. Li H, et al. J Virol. 2011 Nov;85(21):11007-15. doi: 10.1128/JVI.05346-11. Epub 2011 Sep 14. J Virol. 2011. PMID: 21917969 Free PMC article. - Optimizing vaccine-induced CD8(+) T-cell immunity: focus on recombinant adenovirus vectors.
Bassett JD, Swift SL, Bramson JL. Bassett JD, et al. Expert Rev Vaccines. 2011 Sep;10(9):1307-19. doi: 10.1586/erv.11.88. Expert Rev Vaccines. 2011. PMID: 21919620 Review. - Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIV(gpe) recombinant vaccine result in gag-specific CD8(+) T-cell responses in mucosal tissues of macaques.
Stevceva L, Alvarez X, Lackner AA, Tryniszewska E, Kelsall B, Nacsa J, Tartaglia J, Strober W, Franchini G. Stevceva L, et al. J Virol. 2002 Nov;76(22):11659-76. doi: 10.1128/jvi.76.22.11659-11676.2002. J Virol. 2002. PMID: 12388726 Free PMC article. - T cell responses to control fungal infection in an immunological memory lens.
Sharma J, Mudalagiriyappa S, Nanjappa SG. Sharma J, et al. Front Immunol. 2022 Sep 13;13:905867. doi: 10.3389/fimmu.2022.905867. eCollection 2022. Front Immunol. 2022. PMID: 36177012 Free PMC article. Review.
Cited by
- Viral-vectored boosting of OmcB- or CPAF-specific T-cell responses fail to enhance protection from Chlamydia muridarum in infection-immune mice and elicits a non-protective CD8-dominant response in naïve mice.
Poston TB, Girardi J, Polson AG, Bhardwaj A, Yount KS, Jaras Salas I, Trim LK, Li Y, O'Connell CM, Leahy D, Harris JM, Beagley KW, Goonetilleke N, Darville T. Poston TB, et al. Mucosal Immunol. 2024 Oct;17(5):1005-1018. doi: 10.1016/j.mucimm.2024.06.012. Epub 2024 Jul 3. Mucosal Immunol. 2024. PMID: 38969067 Free PMC article. - Recombinant single-cycle influenza virus with exchangeable pseudotypes allows repeated immunization to augment anti-tumour immunity with immune checkpoint inhibitors.
Kandasamy M, Gileadi U, Rijal P, Tan TK, Lee LN, Chen J, Prota G, Klenerman P, Townsend A, Cerundolo V. Kandasamy M, et al. Elife. 2023 Jan 10;12:e76414. doi: 10.7554/eLife.76414. Elife. 2023. PMID: 36626205 Free PMC article. - Multiple modes of antigen exposure induce clonotypically diverse epitope-specific CD8+ T cells across multiple tissues in nonhuman primates.
Simpson J, Starke CE, Ortiz AM, Ransier A, Darko S, Douek DC, Brenchley JM. Simpson J, et al. PLoS Pathog. 2022 Jul 7;18(7):e1010611. doi: 10.1371/journal.ppat.1010611. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35797339 Free PMC article. - Adenovirus vector-based vaccine for infectious diseases.
Sakurai F, Tachibana M, Mizuguchi H. Sakurai F, et al. Drug Metab Pharmacokinet. 2022 Feb;42:100432. doi: 10.1016/j.dmpk.2021.100432. Epub 2021 Nov 12. Drug Metab Pharmacokinet. 2022. PMID: 34974335 Free PMC article. Review. - Respiratory and Intramuscular Immunization With ChAdOx2-NPM1-NA Induces Distinct Immune Responses in H1N1pdm09 Pre-Exposed Pigs.
Vatzia E, Allen ER, Manjegowda T, Morris S, McNee A, Martini V, Kaliath R, Ulaszewska M, Boyd A, Paudyal B, Carr VB, Chrun T, Maze E, MacLoughlin R, van Diemen PM, Everett HE, Lambe T, Gilbert SC, Tchilian E. Vatzia E, et al. Front Immunol. 2021 Nov 3;12:763912. doi: 10.3389/fimmu.2021.763912. eCollection 2021. Front Immunol. 2021. PMID: 34804053 Free PMC article.
References
- Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. - PubMed
- Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. - PubMed
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007387/AI/NIAID NIH HHS/United States
- R01 AI066924/AI/NIAID NIH HHS/United States
- U19 AI066305-01/AI/NIAID NIH HHS/United States
- U19 AI066305/AI/NIAID NIH HHS/United States
- T32 AI07387/AI/NIAID NIH HHS/United States
- U19 AI078526/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials