Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA - PubMed (original) (raw)
Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA
Jing Qiao et al. Am J Physiol Cell Physiol. 2008 Nov.
Abstract
The cAMP-PKA cascade is a recognized signaling pathway important in inhibition of inflammatory injury events such as endothelial permeability and leucocyte trafficking, and a critical target of regulation is believed to be inhibition of Rho proteins. Here, we hypothesize that PKA directly phosphorylates GTP dissociation inhibitor (GDI) to negatively regulate Rho activity. Amino acid analysis of GDIalpha showed two potential protein kinase A (PKA) phosphorylation motifs, Ser(174) and Thr(182). Using in vitro kinase assay and mass spectrometry, we found that the purified PKA catalytic subunit phosphorylated GDIalpha-GST fusion protein and PKA motif-containing GDIalpha peptide at Ser(174), but not Thr(182). Transfection of COS-7 cells with mutated full-length GDIalpha at Ser(174) to Ala(174) (GDIalpha-Ser(174A)) abrogated the ability of cAMP to phosphorylate GDIalpha. However, mutation of Thr(182) to Ala(182) (GDIalpha-Thr(182A)) did not abrogate, and cAMP increased phosphorylation of GDIalpha to a similar extent as wild-type GDIalpha transfectants. The mutant GDIalpha-Ser(174A), but not GDIalpha-Thr(182A), was unable to prevent cAMP-mediated inhibition of Rho-dependent serum-response element reporter activity. Furthermore, the mutant GDIalpha-Ser(174A) was unable to prevent the thrombin-induced RhoA activation. Coprecipitation studies indicated that neither mutation of the PKA consensus sites nor phosphorylation alter GDIalpha binding with RhoA, suggesting that phosphorylation of Ser(174) regulated preformed GDIalpha-RhoA complexes. The findings provide strong support that the selective phosphorylation at Ser(174) by PKA is a signaling pathway in the negative regulation of RhoA activity and therefore could be a potential protective mechanism for inflammatory injury.
Figures
Fig. 1.
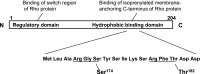
Putative PKA phosphorylation sites on GTP dissociation inhibitor α (GDIα). Two potential PKA consensus phosphorylation sites (underlined) are located in the hydrophobic binding domain of GDIα: Arg-Gly-Ser (amino acids 172-174) and Arg-Phe-Thr (amino acids 180-182). Additionally, two GDI regions bind Rho proteins: NH2-terminal regulatory domain binds the switch region of Rho; COOH-terminal hydrophobic binding domain binds the isoprenylated membrane-anchoring region of Rho.
Fig. 2.
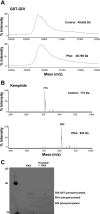
Purified PKA phosphorylates GDIα-GST. Representative mass spectrometric graphs of molecular mass of purified GDIα-GST (A) and Kemptide (positive PKA substrate control, B), n = 3; C: phosphorylation detected by Pro-Q Diamond Phosphoprotein Gel staining Kit (lane 1 = molecular mass standards; lane 2 = GDIα-GST uncleaved; lane 3 = GDIα-GST cleaved with human α-thrombin, 10−6 μM); purified PKA catalytic subunit = 25 units; control, absence of PKA.
Fig. 3.
Purified PKA phosphorylates motif-containing GDIα peptides. Representative mass spectrometric graphs of molecular mass of purified GDIα peptides containing PKA phosphorylation consensus sites: GDIα peptideS174 (n = 3) (A), GDIα peptideT182 (n = 3) (B), GDIα peptideS174/T182 (n = 5) (C), and control peptideA174/A182 (n = 5) (D); PKA = 100 units PKA catalytic subunit; control = absence of PKA.
Fig. 4.
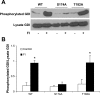
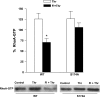
Mutation of GDIα-Ser174 abrogates cAMP-mediated phosphorylation. COS-7 cells were transfected with wild-type GDIα (wt) or the mutant constructs GDIα-Ser174A (S174A) or GDIα-Thr182A (T182A) overnight and treated with 20 μM forskolin and 2 μM IBMX (FI) to increase intracellular level of cAMP. GDIα phosphorylation was determined using the PhosphoProtein purification kit from Qiagen (
materials and methods
). A: top, representative Western blot showing detection of GDIα phosphorylation from the transfectants; bottom, total GDIα in cell lysates. B: bar graph summarizes results from 6 separate determinations; *P < 0.01 compared with control.
Fig. 5.
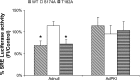
Mutation of GDIα-Ser174 prevents cAMP-induced inhibition of Rho-dependent activity. COS-7 cells were cotransfected overnight with the Rho-dependent SRE-luciferase reporter plasmid and with either wtGDIα (wt), GDIα-Ser174A (S174A), or GDIα-Thr182A (T182A). For PKA specificity, cells were overexpressed with the recombinant adenovirus containing PKI inhibitor gene; AdNull served as control (see
materials and methods
). One group was treated with FI for 8 h to increase intracellular cAMP levels, whereas another remained as untreated control. Luciferase activity was measured in all cell groups, and results are reported as %FI/control; n = 10–14 separate determinations. *P < 0.01 compare with nontreated control in wtGDIα and GDIα-Thr182A-transfected groups.
Fig. 6.
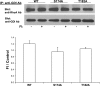
Mutation of GDIα-Ser174 prevented the ability of cAMP to inhibit mediator-induced RhoA activation. COS-7 cells were transfected with wtGDIα (wt) or GDIαS174A (S174A) overnight. Controls and those treated with FI for 30 min were stimulated with human α-thrombin (Thr; 100 nM for 10 min). RhoA activation was determined by affinity-binding assay (see
materials and methods
), and densitometric scans of bands are summarized in the bar graph. A representative Western blot of the pull-downed RhoA-GTP is shown. Values are reported as means ± SE of normalized RhoA-GTP; n = 5. *P < 0.01.
Fig. 7.
Phosphorylation of GDIα-Ser174 did not alter GDIα-RhoA complex formation. Coprecipitation analysis were determined from COS-7 cells transfected with wtαGDI (wt), GDIα-Ser174A (S174A), or GDIα-Thr182A (T182A) overnight, followed by treatment with FI to increase intracellular levels of cAMP. Top: affinity-purified anti-GDI Ab was used for immunoprecipitation, and separated proteins were detected by Western blot analysis with anti-RhoA or anti-GDI antibody; a representative Western blot from five separate determinations. Bottom: bar graph summarization of densitometric scans of bands.
Similar articles
- Serine phosphorylation negatively regulates RhoA in vivo.
Ellerbroek SM, Wennerberg K, Burridge K. Ellerbroek SM, et al. J Biol Chem. 2003 May 23;278(21):19023-31. doi: 10.1074/jbc.M213066200. Epub 2003 Mar 24. J Biol Chem. 2003. PMID: 12654918 - GDI-1 phosphorylation switch at serine 96 induces RhoA activation and increased endothelial permeability.
Knezevic N, Roy A, Timblin B, Konstantoulaki M, Sharma T, Malik AB, Mehta D. Knezevic N, et al. Mol Cell Biol. 2007 Sep;27(18):6323-33. doi: 10.1128/MCB.00523-07. Epub 2007 Jul 16. Mol Cell Biol. 2007. PMID: 17636025 Free PMC article. - cAMP/PKA antagonizes thrombin-induced inactivation of endothelial myosin light chain phosphatase: role of CPI-17.
Aslam M, Härtel FV, Arshad M, Gündüz D, Abdallah Y, Sauer H, Piper HM, Noll T. Aslam M, et al. Cardiovasc Res. 2010 Jul 15;87(2):375-84. doi: 10.1093/cvr/cvq065. Epub 2010 Mar 3. Cardiovasc Res. 2010. PMID: 20202976 - PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction.
Qiao J, Huang F, Lum H. Qiao J, et al. Am J Physiol Lung Cell Mol Physiol. 2003 Jun;284(6):L972-80. doi: 10.1152/ajplung.00429.2002. Epub 2003 Feb 14. Am J Physiol Lung Cell Mol Physiol. 2003. PMID: 12588708 - The 'invisible hand': regulation of RHO GTPases by RHOGDIs.
Garcia-Mata R, Boulter E, Burridge K. Garcia-Mata R, et al. Nat Rev Mol Cell Biol. 2011 Jul 22;12(8):493-504. doi: 10.1038/nrm3153. Nat Rev Mol Cell Biol. 2011. PMID: 21779026 Free PMC article. Review.
Cited by
- ZNF185 prevents stress fiber formation through the inhibition of RhoA in endothelial cells.
Suzuki S, Ando F, Kitagawa S, Hara Y, Fujiki T, Mandai S, Susa K, Mori T, Sohara E, Rai T, Uchida S. Suzuki S, et al. Commun Biol. 2023 Jan 11;6(1):29. doi: 10.1038/s42003-023-04416-x. Commun Biol. 2023. PMID: 36631535 Free PMC article. - RhoA-mediated potential regulation of blood-tumor barrier permeability by bradykinin.
Ma T, Xue Y. Ma T, et al. J Mol Neurosci. 2010 Sep;42(1):67-73. doi: 10.1007/s12031-010-9345-x. Epub 2010 Apr 6. J Mol Neurosci. 2010. PMID: 20369389 - Mini-review: novel therapeutic strategies to blunt actions of pneumolysin in the lungs.
Lucas R, Czikora I, Sridhar S, Zemskov E, Gorshkov B, Siddaramappa U, Oseghale A, Lawson J, Verin A, Rick FG, Block NL, Pillich H, Romero M, Leustik M, Schally AV, Chakraborty T. Lucas R, et al. Toxins (Basel). 2013 Jul 15;5(7):1244-60. doi: 10.3390/toxins5071244. Toxins (Basel). 2013. PMID: 23860351 Free PMC article. Review. - A PKA/cdc42 Signaling Axis Restricts Angiogenic Sprouting by Regulating Podosome Rosette Biogenesis and Matrix Remodeling.
MacKeil JL, Brzezinska P, Burke-Kleinman J, Craig AW, Nicol CJB, Maurice DH. MacKeil JL, et al. Sci Rep. 2019 Feb 20;9(1):2385. doi: 10.1038/s41598-018-37805-y. Sci Rep. 2019. PMID: 30787359 Free PMC article. - 14-3-3τ promotes breast cancer invasion and metastasis by inhibiting RhoGDIα.
Xiao Y, Lin VY, Ke S, Lin GE, Lin FT, Lin WC. Xiao Y, et al. Mol Cell Biol. 2014 Jul;34(14):2635-49. doi: 10.1128/MCB.00076-14. Mol Cell Biol. 2014. PMID: 24820414 Free PMC article.
References
- Adamson P, Etienne S, Couraud PO, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. J Immunol 162: 2964–2973, 1999. - PubMed
- Anwar KN, Fazal F, Malik AB, Rahman A. RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65. J Immunol 173: 6965–6972, 2004. - PubMed
- Balasubramanian N, Levay K, Keren-Raifman T, Faurobert E, Slepak VZ. Phosphorylation of the regulator of G protein signaling RGS9-1 by protein kinase A is a potential mechanism of light- and Ca2+-mediated regulation of G protein function in photoreceptors. Biochemistry 40: 12619–12627, 2001. - PubMed
- Balyasnikova IV, Pelligrino DA, Greenwood J, Adamson P, Dragon S, Raza H, Galea E. Cyclic adenosine monophosphate regulates the expression of the intercellular adhesion molecule and the inducible nitric oxide synthase in brain endothelial cells. J Cereb Blood Flow Metab 20: 688–699, 2000. - PubMed
- Barnard JW, Seibert AF, Prasad VR, Smart DA, Strada SJ, Taylor AE, Thompson WJ. Reversal of pulmonary capillary ischemia-reperfusion injury by rolipram, a cAMP phosphodiesterase inhibitor. J Appl Physiol 77: 774–781, 1994. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials