Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod - PubMed (original) (raw)
Cyclical and dose-dependent responses of adult human mature oligodendrocytes to fingolimod
Veronique E Miron et al. Am J Pathol. 2008 Oct.
Abstract
Fingolimod is a sphingosine-1-phosphate (S1P) analogue that has been used in clinical trials as a systemic immunomodulatory therapy for multiple sclerosis. Fingolimod readily accesses the central nervous system, raising the issue of its direct effects on neural cells. We assessed the effects of active fingolimod on dissociated cultures of mature, myelin-producing oligodendrocytes (OLGs) derived from adult human brain. Human OLGs express S1P receptor transcripts in relative abundance of S1P5>S1P3>S1P1, with undetectable levels of S1P4. Low doses of fingolimod (100 pmol/L to 1 nmol/L) induced initial membrane elaboration (2 days), subsequent retraction (4 days), and recurrence of extension with prolonged treatment (8 days). Higher doses (10 nmol/L to 1 mumol/L) caused the opposite modulation of membrane dynamics. Retraction was rescued by co-treatment with the S1P3/S1P5 pathway antagonist, suramin, and was associated with RhoA-mediated cytoskeletal signaling. Membrane elaboration was mimicked using the S1P1 agonist SEW2871. Fingolimod rescued human OLGs from serum and glucose deprivation-induced apoptosis, which was reversed with suramin co-treatment and mimicked using an S1P5 agonist. High doses of fingolimod induced an initial down-regulation of S1P5 mRNA levels relative to control (4 hours), subsequent up-regulation (2 days), and recurrent down-regulation (8 days). S1P1 mRNA levels were inversely regulated compared with S1P5. These results indicate that fingolimod modulates maturity- and species-specific OLG membrane dynamics and survival responses that are directly relevant for myelin integrity.
Figures
Figure 1
Human adult mature OLGs have transcripts for sphingosone 1-phosphate (S1P) receptors. qPCR levels for S1P receptors were normalized to β-actin levels in the respective sample and then normalized to control levels. Human mature OLGs have S1P receptor transcripts in the relative abundance of S1P5>S1P1>S1P3, and undetectable S1P4.
Figure 2
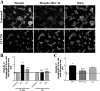
Fingolimod regulates human mature OLG cytoskeleton dynamics in a dose- and treatment duration-dependent manner. A: Representative images of cultures immunostained against MAG. Untreated cultures showed maintenance of membrane elaboration throughout time (left). Treatment with a low dose of fingolimod-phosphate (100 pmol/L, middle) induced an initial membrane elaboration (2 days), subsequent retraction (4 days), and recurrent extension (8 days). Conversely, treatment with a high dose of fingolimod-phosphate (100 nmol/L, right) induced initial membrane retraction (2 days), subsequent elaboration (4 days), and ensuing retraction (8 days). B: Quantification of membrane elaboration expressed as mean area of MAG staining per cell (μm2/cell) normalized to untreated culture values at the respective time point. Treatment with low doses of fingolimod-phosphate (100 pmol/L to 1 nmol/L) induced a cyclic regulation of membrane elaboration; treatment with higher doses (100 nmol/L to 1 μmol/L) provoked a reciprocal modulation of membrane dynamics. *P < 0.05 relative to control; δ_P_ < 0.05 relative to previous time point. C: Quantification of cytoskeletal modulation expressed as mean area of phalloidin staining per cell (μm2/cell) normalized to untreated culture values at the respective time point. *P < 0.05, **P < 0.01 relative to control; δ_P_ < 0.05, δδ_P_ < 0.01, δδδ_P_ < 0.001 relative to previous time point. Results confirm those obtained from quantification of area of MAG staining and suggest modifications at the level of the actin cytoskeleton. D: Co-treatment of cultures with the S1P3/5 pathway antagonist, suramin (100 nmol/L), antagonized the process retraction observed with 1 μmol/L fingolimod-phosphate at 2 days of treatment. Treatment with the S1P1-specific agonist, SEW2871 (100 nmol/L), for 2 days induced significant membrane elaboration relative to control. *P < 0.05 relative to control; θ_P_ < 0.05 relative to fingolimod alone. Scale bar = 50 μm.
Figure 3
Fingolimod-induced activation of a cytoskeletal modulator in mature OLGs indicative of functional signaling downstream of S1P receptors. A: Representative images of untreated (top) and fingolimod-phosphate-treated (FTY, 1 μmol/L; bottom) mature OLG cultures immunostained for the nuclear stain Hoechst (left), phospho-myosin light chain (MLC) II (middle), and MAG, demonstrate that at 15 minutes of treatment fingolimod-phosphate increases the intensity of phospho-MLC II staining in OLGs relative to control. B: Quantification of phospho-MLC II staining intensity per cell normalized to untreated control at the respective time point. fingolimod-phosphate (1 μmol/L) and the positive control LPA induce a significant increase in phospho-MLC II staining intensity at 15 minutes, with an expected drop at a later time point (1 hour). ***P < 0.001 relative to control; δδδ_P_ < 0.001 relative to previous time point; δδ_P_ < 0.01 relative to previous time point. C: The fingolimod-induced increase in intensity of phospho-MLC II staining per cell at 15 minutes was negated by co-treatment with the S1P3/5 pathway antagonist, suramin (sur, 100 nmol/L), whereas the increase in intensity was still observed when suramin was applied alone. *P < 0.05, **P < 0.01 relative to control; θθθ_P_ < 0.001 relative to fingolimod-phosphate alone.
Figure 4
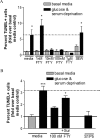
Fingolimod rescues human adult mature OLGs from serum and glucose deprivation-induced cell death. A: Deprivation of culture supplements for 4 days (black) significantly increased the proportion of apoptotic TUNEL-positive mature OLGs to 6.2 ± 1.7-fold greater than basal media levels (gray). A low dose of fingolimod-phosphate (FTY, 1 nmol/L) was unable to rescue OLGs from apoptosis. Higher doses of fingolimod-phosphate (10 nmol/L to 1 μmol/L) significantly decreased the percentage of apoptotic cells to control levels. Treatment with the S1P1-specific agonist, SEW23871 (SEW, 100 nmol/L) was not sufficient in mimicking this rescue effect. *P < 0.05 relative to basal media condition. B: The prosurvival effect of fingolimod-phosphate was antagonized by co-treatment with the S1P3/5 pathway antagonist, suramin (100 nmol/L) and was mimicked by a S1P5-specific agonist (10 nmol/L). ***P < 0.001 and *P < 0.05 relative to basal media condition.
Figure 5
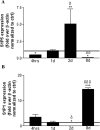
Fingolimod reciprocally modulates S1P1 and S1P5 receptor transcripts in human adult mature OLGs in a cyclic manner. qPCR results for S1P5 (A) and S1P1 (B) were normalized to β-actin in the respective sample and normalized to untreated cultures at the respective time point. At 4 hours of treatment with fingolimod-phosphate (1 μmol/L), S1P5 is down-regulated and S1P1 is concomitantly up-regulated relative to control. By 2 days of treatment, S1P1 is down-regulated and S1P5 is up-regulated. The opposite is observed at 8 days of treatment. At all time points, S1P3 levels remained low and S1P4 remained undetectable. S1P receptor levels were progressively decreased throughout time in untreated cultures likely because of the presence of S1P in the serum-supplemented media. *P < 0.05 relative to control; δ_P_ < 0.05, δδ_P_ < 0.01, δδδ_P_ < 0.001 relative to previous time point.
Similar articles
- FTY720 modulates human oligodendrocyte progenitor process extension and survival.
Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. Miron VE, et al. Ann Neurol. 2008 Jan;63(1):61-71. doi: 10.1002/ana.21227. Ann Neurol. 2008. PMID: 17918267 - Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices.
Miron VE, Ludwin SK, Darlington PJ, Jarjour AA, Soliven B, Kennedy TE, Antel JP. Miron VE, et al. Am J Pathol. 2010 Jun;176(6):2682-94. doi: 10.2353/ajpath.2010.091234. Epub 2010 Apr 22. Am J Pathol. 2010. PMID: 20413685 Free PMC article. - Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy.
Groves A, Kihara Y, Chun J. Groves A, et al. J Neurol Sci. 2013 May 15;328(1-2):9-18. doi: 10.1016/j.jns.2013.02.011. Epub 2013 Mar 19. J Neurol Sci. 2013. PMID: 23518370 Free PMC article. Review. - Non-phosphorylated FTY720 induces apoptosis of human microglia by activating SREBP2.
Yoshino T, Tabunoki H, Sugiyama S, Ishii K, Kim SU, Satoh J. Yoshino T, et al. Cell Mol Neurobiol. 2011 Oct;31(7):1009-20. doi: 10.1007/s10571-011-9698-x. Epub 2011 Apr 26. Cell Mol Neurobiol. 2011. PMID: 21519925 Free PMC article. - Central effects of fingolimod.
Cruz VT, Fonseca J. Cruz VT, et al. Rev Neurol. 2014 Aug 1;59(3):121-8. Rev Neurol. 2014. PMID: 25030072 Review. English, Spanish.
Cited by
- Novel Potent Selective Orally Active S1P5 Receptor Antagonists.
Ma B, Guckian KM, Liu XG, Yang C, Li B, Scannevin R, Mingueneau M, Drouillard A, Walzer T. Ma B, et al. ACS Med Chem Lett. 2021 Jan 15;12(3):351-355. doi: 10.1021/acsmedchemlett.0c00631. eCollection 2021 Mar 11. ACS Med Chem Lett. 2021. PMID: 33738061 Free PMC article. - Heterogeneity of oligodendrocyte progenitor cells in adult human brain.
Leong SY, Rao VT, Bin JM, Gris P, Sangaralingam M, Kennedy TE, Antel JP. Leong SY, et al. Ann Clin Transl Neurol. 2014 Apr;1(4):272-83. doi: 10.1002/acn3.55. Epub 2014 Mar 26. Ann Clin Transl Neurol. 2014. PMID: 25590039 Free PMC article. - [Oral fingolimod in multiple sclerosis: therapeutic modulation of the sphingosine-1-phosphate system].
Aktas O, Ingwersen J, Kieseier B, Küry P, Hohlfeld R, Hartung HP. Aktas O, et al. Nervenarzt. 2011 Feb;82(2):215-25. doi: 10.1007/s00115-010-3075-8. Nervenarzt. 2011. PMID: 20842337 Review. German. - Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma.
Estrada-Bernal A, Palanichamy K, Ray Chaudhury A, Van Brocklyn JR. Estrada-Bernal A, et al. Neuro Oncol. 2012 Apr;14(4):405-15. doi: 10.1093/neuonc/nos005. Epub 2012 Feb 20. Neuro Oncol. 2012. PMID: 22351749 Free PMC article. - Siponimod ameliorates metabolic oligodendrocyte injury via the sphingosine-1 phosphate receptor 5.
Behrangi N, Heinig L, Frintrop L, Santrau E, Kurth J, Krause B, Atanasova D, Clarner T, Fragoulis A, Joksch M, Rudolf H, Meuth SG, Joost S, Kipp M. Behrangi N, et al. Proc Natl Acad Sci U S A. 2022 Oct 4;119(40):e2204509119. doi: 10.1073/pnas.2204509119. Epub 2022 Sep 26. Proc Natl Acad Sci U S A. 2022. PMID: 36161894 Free PMC article.
References
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci. 2005;6:683–690. - PubMed
- Smith ME. The turnover of myelin in the adult rat. Biochim Biophys Acta. 1968;164:285–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources