NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes - PubMed (original) (raw)
NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes
Rodney E Infante et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Egress of lipoprotein-derived cholesterol from lysosomes requires two lysosomal proteins, polytopic membrane-bound Niemann-Pick C1 (NPC1) and soluble Niemann-Pick C2 (NPC2). The reason for this dual requirement is unknown. Previously, we showed that the soluble luminal N-terminal domain (NTD) of NPC1 (amino acids 25-264) binds cholesterol. This NTD is designated NPC1(NTD). We and others showed that soluble NPC2 also binds cholesterol. Here, we establish an in vitro assay to measure transfer of [(3)H]cholesterol between these two proteins and phosphatidylcholine liposomes. Whereas NPC2 rapidly donates or accepts cholesterol from liposomes, NPC1(NTD) acts much more slowly. Bidirectional transfer of cholesterol between NPC1(NTD) and liposomes is accelerated >100-fold by NPC2. A naturally occurring human mutant of NPC2 (Pro120Ser) fails to bind cholesterol and fails to stimulate cholesterol transfer from NPC1(NTD) to liposomes. NPC2 may be essential to deliver or remove cholesterol from NPC1, an interaction that links both proteins to the cholesterol egress process from lysosomes. These findings may explain how mutations in either protein can produce a similar clinical phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
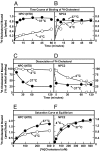
Fig. 1.
Kinetics of [3H]cholesterol binding to purified NPC proteins. (A and B) Time course at different temperatures. Each reaction, in a final volume of 80 μl of buffer B (pH 6.5) with 0.004% Nonidet P-40, contained 4 pmol of NPC1(NTD)-LVPRGS-His-8-FLAG (A) or 8 pmol of NPC2-His-10 (B) and 100 nM [3H]cholesterol (132 × 103 dpm/pmol). After incubation for indicated time at 4°C (○) or 37°C (●), the amount of bound [3H]cholesterol was measured with the Ni-NTA-agarose binding assay. Each value is the average of duplicate assays and represents total binding after subtraction of a blank value (0.01–0.02 pmol per tube). (C and D) Dissociation of previously bound [3H]cholesterol from NPC proteins at different temperatures. [3H]Cholesterol complexed to NPC1(NTD)-LVPRGS-His-8-FLAG (C) or NPC2-His-10 (D) at 4°C was isolated by gel filtration. Fractions containing NPC1 or NPC2 complexed to [3H]cholesterol were pooled (3 ml), diluted 7-fold with buffer B (pH 6.5) containing 0.004% Nonidet P-40 and 10 μM unlabeled cholesterol, and incubated at 4°C (○) or 37°C (●). At the indicated time, a 1-ml-aliquot of the pooled 21-ml sample was transferred to a tube containing 600 μl of Ni-NTA-agarose beads. After incubation for 3 min at 4°C, the beads were centrifuged at 600_g_ for 1 min, after which the supernatant was assayed for radioactivity. Each value is the average of duplicate assays and represents the percentage of [3H]cholesterol remaining bound to the beads relative to zero-time value. The “100% initial binding” values at zero time for NPC1(NTD) and NPC2 were 1.6 and 0.24 pmol per tube, respectively. (E and F) Saturation curves for equilibrium binding at different temperatures. This experiment was carried out as in A and B except that [3H]cholesterol concentration varied as indicated, and incubation time at 4°C was 20 h for NPC1(NTD) and 45 min for NPC2 and at 37°C, 45 min for both proteins.
Fig. 2.
Transfer of [3H]cholesterol from donor NPC protein to acceptor NPC protein. Schematic diagrams of the two transfer assays are shown at the top of each column. (A–D) Time course at different temperatures. Each reaction, in a final volume of 100 μl of buffer B (pH 5.5) with 0.004% Nonidet P-40, contained ≈40 pmol of NPC1(NTD)-LVPR (A and C) or NPC2-FLAG (B and D) each complexed to [3H]cholesterol (1.3–1.9 pmol; 132 × 103 dpm/pmol) and 200 pmol of NPC1(NTD)-LVPRGS-His-8-FLAG (○) or NPC2-His-10 (●). After incubation for indicated time at 4°C (A and B) or 37°C (C and D), the amount of [3H]cholesterol transferred to the indicated acceptor His-tagged NPC protein was measured with the Ni-NTA-agarose cholesterol transfer assay. Each value represents the percentage of [3H]cholesterol transferred. The 100% values for transfer from donor were 1.9 pmol (A), 1.5 (B and C), and 1.3 (D). (E and F) Transfer at 4°C as a function of the amount of acceptor NPC protein. This experiment was carried out as in A–D except that the amount of acceptor NPC protein varied as indicated and time of incubation at 4°C was 30 min. The 100% values for transfer of [3H]cholesterol from NPC1(NTD) and NPC2 were 2.0 and 1.2 pmol, respectively. (G and H) Transfer at 4°C as a function of pH. Each reaction in a final volume of 300 μl of buffer A, B, or E with 0.004% Nonidet P-40 at the indicated pH, contained ≈30 pmol of NPC1(NTD)-LVPR (G) or NPC2-FLAG (H) each complexed to [3H]cholesterol (1.6 and 1.0 pmol, respectively) and 200 pmol of either NPC1(NTD)-LVPRGS-His-8-FLAG (○) or NPC2-His-10 (●). After incubation for 30 min at 4°C, the amount of [3H]cholesterol transferred to the indicated acceptor His-tagged NPC was measured as described above except samples were diluted with 1.1 ml of buffer A with 0.004% Nonidet P-40. Each value is the average of duplicate assays and represents the percentage of [3H]cholesterol transferred. The 100% values for transfer from NPC1(NTD) and NPC2 were 1.6 and 1.0 pmol, respectively.
Fig. 3.
Transfer of [3H]cholesterol from donor NPC protein to acceptor PC liposomes. Schematic diagrams of the two transfer assays are shown at the top of each column. (A–D) Time course at different temperatures. Each reaction, in a final volume of 200 μl of buffer B (pH 5.5), contained ≈30 pmol of NPC1(NTD)-LVPRGS-His-8-FLAG (A and C) or NPC2-His-10 (B and D) each complexed to [3H]cholesterol (1.0 and 0.3 pmol, respectively; 132 × 103 dpm/pmol) and 60 μg of PC liposomes labeled with Texas red dye in the absence (○) or presence (●) of 100 pmol NPC2-His-10 (A and C) or NPC1(NTD)-LVPRGS-His-8-FLAG (B and D). After incubation for the indicated time at 4°C (A and B) or 37°C (C and D), the amount of [3H]cholesterol transferred to liposomes was measured in the Ni-NTA-agarose cholesterol transfer assay. Each value is the average of duplicate assays and represents the percentage of [3H]cholesterol transferred to liposomes. The 100% values for transfer from NPC1(NTD) and NPC2 were 1.0 and 0.3 pmol, respectively. Blank values in the absence of liposomes (1–4%) were subtracted. (E and F) Transfer at 4°C as a function of the amount of liposomes. This experiment was carried out as in A–D except that the amount of liposomes varied as indicated, and the time of incubation at 4°C was 30 min. The 100% values for transfer of [3H]cholesterol from NPC1(NTD) and NPC2 were 0.4 and 1.7 pmol, respectively. Blank values in the absence of liposomes (2–5%) were subtracted. (G and H) Transfer at 4°C as a function of pH. The conditions for this experiment are the same as those in E and F except that the final volume of the reaction was 300 μl in buffer A, B, or E at the indicated pH, and the acceptor was 60 μg of PC liposomes in the absence (○) or presence (●) of 100 pmol of NPC2-His-10 (G) or 100 pmol NPC1(NTD)-LVPRGS-His-8-FLAG (H). The 100% values for transfer of [3H]cholesterol from NPC1(NTD) and NPC2 were 0.8 and 0.5 pmol, respectively. Blank values in absence of liposomes (1–5%) were subtracted.
Fig. 4.
Mutant NPC2 fails to transfer [3H]cholesterol from NPC1(NTD) to acceptor PC liposomes. (A) Saturation curves for equilibrium binding of [3H]cholesterol for wild-type and mutant NPC2. Binding reactions were carried out as described in Fig. 1F except that each reaction was incubated for 2 h at 4°C and contained 8 pmol of wild-type (●) or P120S mutant (○) version of NPC2-His-10. Each value is the average of duplicate assays and represents binding after subtraction of blank values (0.01–0.11 pmol). (Inset) Coomassie Brilliant blue R-250 stain of wild-type and mutant NPC2 proteins after electrophoresis on 13% SDS/PAGE (3 μg of each protein loaded on gel). (B) Transfer of [3H]cholesterol from donor NPC1(NTD) to acceptor liposomes as a function of varying concentrations of wild-type or mutant NPC2. Assays were carried out as in Fig. 3 except that each reaction was carried out for 10 min at 4°C and contained ≈50 pmol of NPC1(NTD)-LVPRGS-His-8-FLAG complexed to [3H]cholesterol (1.2 pmol; 132 × 103 dpm/pmol), 60 μg of PC liposomes, and the indicated concentration of wild-type (●) or P120S mutant (○) version of NPC2-His-10. Each value is the average of duplicate assays and represents the percentage of [3H]cholesterol transferred to liposomes. The 100% value for transfer from NPC1(NTD) was 1.2 pmol. Blank values in the absence of NPC2 protein (8%) were subtracted.
Fig. 5.
Transfer of [3H]cholesterol from donor PC liposomes to acceptor NPC protein. Schematic diagrams of the two transfer assays are shown at the top of each column. (A–D) Time course at different temperatures. Each reaction, in a final volume of 200 μl of buffer B (pH 5.5), contained 17 μg of PC liposomes containing 540 pmol of [3H]cholesterol (930 dpm/pmol) labeled with Texas red dye and 100 pmol of NPC1(NTD)-LVPRGS-His-8-FLAG (A and C) or NPC2-His-10 (B and D) in the presence (●) or absence (○) of 100 pmol of either NPC2-FLAG (A and C) or NPC1(NTD)-LVPR (B and D). After incubation for the indicated time at 4°C (A and B) or 37°C (C and D), the amount of [3H]cholesterol transferred to acceptor His-tagged NPC protein was measured in the Ni-NTA-agarose cholesterol transfer assay. Each value is the average of duplicate assays and represents the amount of [3H]cholesterol transferred from liposomes to the indicated acceptor His-tagged NPC protein. Blank values in the absence of His-tagged NPC protein (0.2–0.3 pmol) were subtracted. (E and F) Transfer at 4°C as a function of the concentration of NPC protein. This experiment was carried out as in A–D except that the amount of acceptor NPC1(NTD)-LVPRGS-His-8-FLAG (E) or acceptor NPC2-FLAG (F) varied as indicated, and the time of incubation at 4°C was 30 min. Each value is the average of duplicate assays and represents the amount of [3H]cholesterol transferred from liposomes to the indicated acceptor NPC protein. Blank values in the absence of His-tagged NPC protein (0.2–0.4 pmol) were subtracted. (G and H) Transfer at 4°C as a function of pH. The conditions for this experiment are the same as those in Fig. 3 G and H except that the donor was 17 μg of PC:[3H]cholesterol liposomes (930 dpm/fmol), and the acceptor was 100 pmol of NPC1(NTD)-LVPRGS-His-8-FLAG (G) or NPC2-His-10 (H) in the absence (○) or presence (●) of 100 pmol of either NPC2-FLAG (G) or NPC1(NTD)-LVPR(H). Each value is the average of duplicate assays and represents the amount of [3H]cholesterol transferred from liposomes to the indicated His-tagged NPC protein. Blank values in the absence of His-tagged NPC protein (0.2–0.3 pmol) were subtracted.
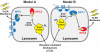
Fig. 6.
Alternative models for the transfer of cholesterol from LDL to lysosomal membranes. The interpretations of Model A and Model B are explained in Discussion.
Comment in
- NPC1/NPC2 function as a tag team duo to mobilize cholesterol.
Subramanian K, Balch WE. Subramanian K, et al. Proc Natl Acad Sci U S A. 2008 Oct 7;105(40):15223-4. doi: 10.1073/pnas.0808256105. Epub 2008 Oct 1. Proc Natl Acad Sci U S A. 2008. PMID: 18832164 Free PMC article. No abstract available.
Similar articles
- Niemann-Pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo.
Goldman SD, Krise JP. Goldman SD, et al. J Biol Chem. 2010 Feb 12;285(7):4983-94. doi: 10.1074/jbc.M109.037622. Epub 2009 Dec 10. J Biol Chem. 2010. PMID: 20007703 Free PMC article. - Identification of surface residues on Niemann-Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes.
Wang ML, Motamed M, Infante RE, Abi-Mosleh L, Kwon HJ, Brown MS, Goldstein JL. Wang ML, et al. Cell Metab. 2010 Aug 4;12(2):166-73. doi: 10.1016/j.cmet.2010.05.016. Cell Metab. 2010. PMID: 20674861 Free PMC article. - Differential trafficking of the Niemann-Pick C1 and 2 proteins highlights distinct roles in late endocytic lipid trafficking.
Zhang M, Sun M, Dwyer NK, Comly ME, Patel SC, Sundaram R, Hanover JA, Blanchette-Mackie EJ. Zhang M, et al. Acta Paediatr Suppl. 2003 Dec;92(443):63-73; discussion 45. doi: 10.1111/j.1651-2227.2003.tb00224.x. Acta Paediatr Suppl. 2003. PMID: 14989468 - Intracellular trafficking of Niemann-Pick C proteins 1 and 2: obligate components of subcellular lipid transport.
Liscum L, Sturley SL. Liscum L, et al. Biochim Biophys Acta. 2004 Oct 11;1685(1-3):22-7. doi: 10.1016/j.bbalip.2004.08.008. Biochim Biophys Acta. 2004. PMID: 15465423 Review. - Function of the Niemann-Pick type C proteins and their bypass by cyclodextrin.
Vance JE, Peake KB. Vance JE, et al. Curr Opin Lipidol. 2011 Jun;22(3):204-9. doi: 10.1097/MOL.0b013e3283453e69. Curr Opin Lipidol. 2011. PMID: 21412152 Review.
Cited by
- Proteomic analysis of mouse models of Niemann-Pick C disease reveals alterations in the steady-state levels of lysosomal proteins within the brain.
Sleat DE, Wiseman JA, Sohar I, El-Banna M, Zheng H, Moore DF, Lobel P. Sleat DE, et al. Proteomics. 2012 Dec;12(23-24):3499-509. doi: 10.1002/pmic.201200205. Epub 2012 Nov 22. Proteomics. 2012. PMID: 23070805 Free PMC article. - The promise and perils of HDAC inhibitors in neurodegeneration.
Didonna A, Opal P. Didonna A, et al. Ann Clin Transl Neurol. 2015 Jan;2(1):79-101. doi: 10.1002/acn3.147. Epub 2014 Dec 3. Ann Clin Transl Neurol. 2015. PMID: 25642438 Free PMC article. Review. - Niemann-Pick Type C Proteins Are Required for Sterol Transport and Appressorium-Mediated Plant Penetration of Colletotrichum orbiculare.
Kodama S, Kajikawa N, Fukada F, Kubo Y. Kodama S, et al. mBio. 2022 Oct 26;13(5):e0223622. doi: 10.1128/mbio.02236-22. Epub 2022 Sep 26. mBio. 2022. PMID: 36154185 Free PMC article. - TDP-43 proteinopathy occurs independently of autophagic substrate accumulation and underlies nuclear defects in Niemann-Pick C disease.
Liu EA, Mori E, Hamasaki F, Lieberman AP. Liu EA, et al. Neuropathol Appl Neurobiol. 2021 Dec;47(7):1019-1032. doi: 10.1111/nan.12738. Epub 2021 Jun 15. Neuropathol Appl Neurobiol. 2021. PMID: 34048071 Free PMC article. - Interorganelle communication, aging, and neurodegeneration.
Petkovic M, O'Brien CE, Jan YN. Petkovic M, et al. Genes Dev. 2021 Apr 1;35(7-8):449-469. doi: 10.1101/gad.346759.120. Genes Dev. 2021. PMID: 33861720 Free PMC article. Review.
References
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
- Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein: Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975;250:8487–8495. - PubMed
- Pentchev PG, Vanier MT, Suzuki K, Patterson MC. In: The Metabolic and Molecular Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2625–2639.
- Pentchev PG. Niemann–Pick C research from mouse to gene. Biochim Biophys Acta. 2004;1685:3–7. - PubMed
- Li AC, Tanaka RD, Callaway K, Fogelman AM, Edwards PA. Localization of 3-hydroxy-3-methylglutaryl CoA reductase and 3-hydroxy-3-methlyglutaryl CoA synthase in the rat liver and intestine is affected by cholestyramine and mevinolin. J Lipid Res. 1988;29:781–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous