Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins - PubMed (original) (raw)
Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins
Stephen R Adams et al. Nat Protoc. 2008.
Abstract
The membrane-permeant fluorogenic biarsenicals FlAsH-EDT(2) and ReAsH-EDT(2) can be prepared in good yields by a straightforward two-step procedure from the inexpensive precursor dyes fluorescein and resorufin, respectively. Handling of toxic reagents such as arsenic trichloride is minimized so the synthesis can be carried out in a typical chemistry laboratory, usually taking about 2-3 d. A wide range of other biarsenical reagents and intermediates that also bind to tetracysteine-tagged (CysCysProGlyCysCys) proteins can be prepared similarly using this general procedure.
Figures
Figure 1
Synthesis of FlAsH-EDT2 and ReAsH-EDT2; (a) HgO, TFA (b) AsCl3, Pd(OAc)2, DIEA, NMP (c) EDT, aqueous acetone.
Figure 2
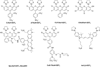
Structures of some other useful biarsenicals reagents and intermediates. CrAsH-EDT2 (refs 4, 11, 17), fluorogenic biarsenicals and intermediate for CaG FlAsH and immobilized biarsenicals; sFlAsH-EDT2 (ref 4) membrane-impermeable fluorogenic biarsenicals; F2-FlAsH (ref 18), photostable fluorogenic biarsenicals; CHoXAsH-EDT2 (refs 4, 17), aggregator for BA-GFP; SpLAsH (ref, colorless and nonfluorescent biarsenicals for targeting other dyes e.g. Alexa594; CaG FlAsH (ref 11), targetable low-affinity Ca2+ indicator; AsCy3 (ref 20), fluorogenic biarsenical based on cyanine dye.
Figure 3
Typical time courses for the reaction of FlAsH-EDT2 (green trace) and ReAsH-EDT2 (red trace) with the tetracysteine peptide, FLNCCPGCCMEP (added at 700 s).
Figure 4
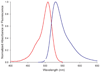
Absorbance (red trace) and fluorescence emission (blue trace) of FlAsH complex with FLNCCPGCCMEP.
Figure 5
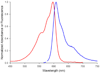
Absorbance (red trace) and fluorescence emission (blue trace) of ReAsH complex with FLNCCPGCCMEP.
Similar articles
- Fluorescent labeling of tetracysteine-tagged proteins in intact cells.
Hoffmann C, Gaietta G, Zürn A, Adams SR, Terrillon S, Ellisman MH, Tsien RY, Lohse MJ. Hoffmann C, et al. Nat Protoc. 2010 Sep;5(10):1666-77. doi: 10.1038/nprot.2010.129. Epub 2010 Sep 23. Nat Protoc. 2010. PMID: 20885379 Free PMC article. - Identification of an orthogonal peptide binding motif for biarsenical multiuse affinity probes.
Chen B, Cao H, Yan P, Mayer MU, Squier TC. Chen B, et al. Bioconjug Chem. 2007 Jul-Aug;18(4):1259-65. doi: 10.1021/bc0603900. Epub 2007 Jun 15. Bioconjug Chem. 2007. PMID: 17569496 - Site-specific labeling of the type 1 ryanodine receptor using biarsenical fluorophores targeted to engineered tetracysteine motifs.
Fessenden JD, Mahalingam M. Fessenden JD, et al. PLoS One. 2013 May 28;8(5):e64686. doi: 10.1371/journal.pone.0064686. Print 2013. PLoS One. 2013. PMID: 23724080 Free PMC article. - Therapeutic and analytical applications of arsenic binding to proteins.
Chen B, Liu Q, Popowich A, Shen S, Yan X, Zhang Q, Li XF, Weinfeld M, Cullen WR, Le XC. Chen B, et al. Metallomics. 2015 Jan;7(1):39-55. doi: 10.1039/c4mt00222a. Epub 2014 Oct 30. Metallomics. 2015. PMID: 25356501 Review. - Exploration of biarsenical chemistry--challenges in protein research.
Pomorski A, Krężel A. Pomorski A, et al. Chembiochem. 2011 May 16;12(8):1152-67. doi: 10.1002/cbic.201100114. Epub 2011 Apr 28. Chembiochem. 2011. PMID: 21538762 Review.
Cited by
- Heme delivery to heme oxygenase-2 involves glyceraldehyde-3-phosphate dehydrogenase.
Dai Y, Fleischhacker AS, Liu L, Fayad S, Gunawan AL, Stuehr DJ, Ragsdale SW. Dai Y, et al. Biol Chem. 2022 Oct 28;403(11-12):1043-1053. doi: 10.1515/hsz-2022-0230. Print 2022 Nov 25. Biol Chem. 2022. PMID: 36302634 Free PMC article. - Tracking the Replication-Competent Zika Virus with Tetracysteine-Tagged Capsid Protein in Living Cells.
Li S, Wang D, Ghulam A, Li X, Li M, Li Q, Ma Y, Wang L, Wu H, Cui Z, Zhang XE. Li S, et al. J Virol. 2022 Apr 13;96(7):e0184621. doi: 10.1128/jvi.01846-21. Epub 2022 Mar 14. J Virol. 2022. PMID: 35285687 Free PMC article. - Engineering of a protein probe with multiple inputs and multiple outputs for evaluation of alpha synuclein aggregation states.
Chau E, Kim JR. Chau E, et al. Biochem Eng J. 2022 Jan;178:108292. doi: 10.1016/j.bej.2021.108292. Epub 2021 Nov 29. Biochem Eng J. 2022. PMID: 35002469 Free PMC article. - Single-molecule FRET and conformational analysis of beta-arrestin-1 through genetic code expansion and a Se-click reaction.
Han MJ, He QT, Yang M, Chen C, Yao Y, Liu X, Wang Y, Zhu ZL, Zhu KK, Qu C, Yang F, Hu C, Guo X, Zhang D, Chen C, Sun JP, Wang J. Han MJ, et al. Chem Sci. 2021 May 31;12(26):9114-9123. doi: 10.1039/d1sc02653d. eCollection 2021 Jul 7. Chem Sci. 2021. PMID: 34276941 Free PMC article. - Bacterial Vivisection: How Fluorescence-Based Imaging Techniques Shed a Light on the Inner Workings of Bacteria.
Cambré A, Aertsen A. Cambré A, et al. Microbiol Mol Biol Rev. 2020 Oct 28;84(4):e00008-20. doi: 10.1128/MMBR.00008-20. Print 2020 Nov 18. Microbiol Mol Biol Rev. 2020. PMID: 33115939 Free PMC article. Review.
References
- Tsien RY. The green fluorescent protein. Annu. Rev Biochem. 1998;67:509–544. - PubMed
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. - PubMed
- Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281:269–272. - PubMed
- Adams SR, et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 2002;124:6063–6076. - PubMed
- Martin BR, Giepmans BN, Adams SR, Tsien RY. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 2005;23:1308–1314. - PubMed
MeSH terms
Substances
Grants and funding
- P20 GM072033-03/GM/NIGMS NIH HHS/United States
- R01 NS027177-15/NS/NINDS NIH HHS/United States
- R01 NS027177-17/NS/NINDS NIH HHS/United States
- P20 GM072033-01/GM/NIGMS NIH HHS/United States
- P20 GM072033-03S1/GM/NIGMS NIH HHS/United States
- R01 NS027177-16/NS/NINDS NIH HHS/United States
- R01 NS027177/NS/NINDS NIH HHS/United States
- R37 NS027177/NS/NINDS NIH HHS/United States
- P20 GM072033-02/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 NS027177-19/NS/NINDS NIH HHS/United States
- P20 GM072033/GM/NIGMS NIH HHS/United States
- P20 GM072033-04/GM/NIGMS NIH HHS/United States
- R01 NS027177-18/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources