Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation - PubMed (original) (raw)
Cripto recruits Furin and PACE4 and controls Nodal trafficking during proteolytic maturation
Marie-Hélène Blanchet et al. EMBO J. 2008.
Abstract
The glycosylphosphatidylinositol (GPI)-anchored proteoglycan Cripto binds Nodal and its type I receptor Alk4 to activate Smad2,3 transcription factors, but a role during Nodal precursor processing has not been described. We show that Cripto also binds the proprotein convertases Furin and PACE4 and localizes Nodal processing at the cell surface. When coexpressed as in early embryonic cells, Cripto and uncleaved Nodal already associated during secretion, and a Cripto-interacting region in the Nodal propeptide potentiated the effect of proteolytic maturation on Nodal signalling. Disruption of the trans-Golgi network (TGN) by brefeldin A blocked secretion, but export of Cripto and Nodal to the cell surface was not inhibited, indicating that Nodal is exposed to extracellular convertases before entering the TGN/endosomal system. Density fractionation and antibody uptake experiments showed that Cripto guides the Nodal precursor in detergent-resistant membranes to endocytic microdomains marked by GFP-Flotillin. We conclude that Nodal processing and endocytosis are coupled in signal-receiving cells.
Figures
Figure 1
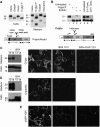
Post-translational modifications of Nodal and Cripto. (A) Western blot of Nodal in lysates (left) and conditioned medium (right) of transfected COS1 cells. Treatment with EndoH or _N_-glycosidase F shifted the size of intracellular Nodal from 42 to 39 kDa, the predicted molecular weight of unmodified precursor. In conditioned medium, precursor and Nodal propeptide (47 and 35 kDa) were sensitive to _N_-glycosidase F and neuraminidase, but not to EndoH. Bottom: impact of processing on the size of Nodal. (B) Western blot analysis of Cripto in COS1 cell lysates before and after treatment with _N_-glycosidase F, EndoH or both, or after incubation of cells with tunicamycin. Processed Cripto lacking the N-terminal 52 amino acids (Minchiotti et al, 2001) has a predicted size of 13 kDa, but migrates at an apparent molecular weight of 18 kDa due to high mannose N-glycosylation. (C) Left: truncated mutant Cripto lacking a GPI signal (CriptoHis) is shifted in size to 29 kDa by complex carbohydrate modifications (Minchiotti et al, 2001), and its release into conditioned medium is blocked by BFA. Right: also shedding of wild-type Cripto is abolished in the presence of BFA. (D) Immunostaining of unpermeabilized COS1 cells shows that BFA blocks the expression of CriptoHis at the cell surface, but not that of wild-type Cripto. (E) Cripto staining at the surface of unpermeabilized cells is blocked in cells where BFA was administered together with cycloheximide.
Figure 2
GPI-anchored Cripto intercepts the Nodal precursor in lipid rafts. (A) In co-transfection assays, Cripto dose-dependently reduces the amount of soluble Flag-tagged Nodal precursor (47 kDa) in conditioned medium (top left panels). Compared with wild-type Nodal, mutant precursor lacking the PC cleavage site (Nr, right panels) is more resistant to Cripto-induced turnover. Bottom panels: GPI-anchored Cripto accumulated in cell lysates. After prolonged exposure, Cripto is also detected in conditioned medium (not shown). The anti-Flag western blot of cell lysates is overexposed to detect fully modified Nodal precursor (47 kDa). (B) Truncated Cripto lacking the GPI anchor (CriptoHis) is released without affecting Nodal. The Nodal propeptide (35 kDa) can be detected in small amounts in conditioned medium, but is below detectable levels in cell lysates (Constam and Robertson, 1999). All results are representative of more than three independent experiments, and the amount of transfected DNA including empty vector in all transfections was 10 μg. γ-Tubulin staining served as a loading control. (C, D) Western blots of detergent extracts of transfected cells fractionated in OptiPrep density gradients. In fraction 1 (lipid rafts), Cripto increases the amount of pre-TGN wild-type precursor (42 kDa) and Nr (right panels in C and D, respectively). The distribution of Cripto was not altered in the absence of Nodal (data not shown). Nr was detected in fraction 1 together with a breakdown product (37 kDa) even in the absence of Cripto (left panels in C and D). Separation of lipid rafts from detergent-soluble proteins was confirmed by re-probing the blots with anti-Caveolin-1 (Cav) and transferrin receptor (Tfr) antibodies. All results are representative of at least three independent experiments.
Figure 3
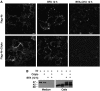
Transport through the TGN/endosomal system is essential for the secretion and processing of Nodal precursor, but not for cell surface expression. (A) Immunostaining of Flag-tagged Nr at the surface of unpermeabilized COS1 cells treated with or without BFA (5 μg/ml). BFA did not prevent accumulation of uncleaved Nodal precursor at the cell surface, except when de novo synthesis was blocked by cycloheximide (CHX, 5 μg/ml). (B) BFA inhibited the secretion of Nodal into the medium, leading to sequestration of the 47-kDa form in cell lysates.
Figure 4
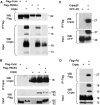
Cripto binds mature and uncleaved Nodal, and a Cripto-interacting region in the propeptide ensures that Nodal processing enhances signalling. (A) Pull down of Flag-tagged Nodal precursor and cleaved fragments (pro, mat) by CriptoHis. Supernatant of 293T cell lines containing Nodal propeptide, mature form and residual uncleaved precursor (bottom panels, input) was incubated with metal agarose beads soaked with (lanes 1 and 2) or without (lanes 3 and 4) CriptoHis. Western blot analysis revealed that CriptoHis (18–28 kDa) precipitates Nodal precursor (pre), together with small amounts of propeptide (pro) and mature form (mat, lane 1). Mutation of the PC recognition motif RQRR′HHL to RQRR′H
LE
in supercleaved Nodal-sc (Constam and Robertson, 1999) increased binding of Cripto to mature Nodal and propeptide (lane 2). Mature Nodal (12 kDa) and precursor (47 kDa) were shifted in size by 6 kDa due to an N-glycosylation site engineered to improve protein stability (Le Good et al, 2005). Nonspecific binding of Nodal to empty beads was below detection (lanes 3 and 4). Input samples in lanes 3 and 4 are identical to those in lanes 1 and 2, respectively. (B) Flag–Nodal (wild type) and Flag–Nsc precursor pulled down wild-type Cripto from detergent-soluble COS1 cell extracts (lanes 1 and 3). Cripto was also precipitated by mature Nodal (Nmat, lane 4) or propeptide (Npro, lane 2), but not in the absence of Nodal (lane 5). Lanes of input samples blotted on the same gel were cut for correct ordering. (C) Coimmunoprecipitation of Cripto3F with Npro–GFP fusion protein. (D) Residues 211–237 of the Nodal propeptide aligned with homologous sequences and other TGFβ family members. Conserved amino acids are indicated in red. Mutated residues (216–223) are indicated in blue. The most highly conserved residues of the CIR are underlined. Rose: conservative substitutions. Green: alternative PC cleavage site of proBmp2/4. (E) Coimmunoprecipitation of secreted CFP–CIR fusion protein with Flag-tagged Cripto. (F) Mutation of four conserved residues in the CIR (CIRmut) reduces Cripto binding. (G) In conditioned medium of transfected 293T cells, NdlΔCIR and NdlCIRmut can be processed independently of Cripto similar to wild-type Nodal (G, top panel). Below: activation of wild-type and CIR-deficient Nodal precursors in 293T cells expressing Cripto and the luciferase reporter pAR3-lux. (H) Mutation of the CIR also inhibits paracrine Nodal/Cripto signalling to 293T cells that were separately transfected with luciferase reporter prior to mixing with Nodal/Cripto expressing cells. Results are representative of two experiments. Error bars indicate standard deviation of triplicate values.
Figure 5
Binding of Cripto to Furin and PACE4. (A) Cripto coprecipitates with Furin or PACE4, but not with control beads. Note that all samples were analysed together on the same gels, but irrelevant lanes were excised where indicated. (B) Reverse pull down of Furin and PACE4 by Cripto confirms the specificity of binding. (C, D) Coimmunoprecipitation of Cripto with the P-domain (Pd) of Furin fused to the C terminus of secreted CFP (C). For reverse pull down, the P-domain was provided with a signal sequence followed by a Flag epitope (D).
Figure 6
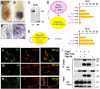
A complex of Cripto and Nodal is activated in signal-receiving cells. (A, B) Whole mount view (A, B) and frozen section (B′) of mouse embryos stained 6.25 days after fertilization for Cripto (A, red) and subsequently for Nodal mRNA (B, purple). Nodal and Cripto transcripts colocalize in the same cells (B'). Nodal transcripts are enriched near cell borders. Scale bars: 20 μm. (C, D) Secreted Nodal in the medium (SN) of transiently transfected 293T cells is completely processed by endogenous PCs both with (+) or without Cripto (C). Nevertheless, induction of the Nodal luciferase reporter pAR3-lux was enhanced up to two-fold if Nodal/Cripto reporter cells were co-cultured with cells overexpressing Furin or PACE4, indicating that Nodal is likely to mature at the cell surface (D). PCs had no effect on reporter cells expressing empty vector (V). (E) For comparison, PCs, Nodal, Cripto and luciferase reporter were co-transfected into a single population of cells and co-cultured with untransfected cells. (F) Immunofluorescence analysis of non-permeabilized COS1 cells showing co-localization of Flag–Nr (green) with Cripto (red). Wild-type (wt) Flag–Nodal was less abundant at the surface of Cripto-transfected cells (G), except when cleavage was blocked by adding the PC inhibitor decanoyl-RVKR-chloromethylketone (H). (I) Cells transfected with Flag–Furin were co-cultured for 24 h with cells expressing Cripto with or without uncleaved Nodal (Nr). The resulting complexes were reversibly crosslinked and immunoprecipitated by anti-Cripto antibody. Anti-Flag immunoblotting showed that Furin was pulled down by a complex of Cripto and uncleaved Nodal precursor. Where indicated by vertical lines, blots were cut to remove one intervening lane.
Figure 7
Cripto recruits Nodal to nonclathrin, noncaveolar endocytic compartments marked by Flotillin-1 and -2. (A) Antibody uptake by Flag-tagged Nodal (red) in early endosomes expressing TfR was undetectable after 5 min irrespective of the presence or absence of Cripto. (B) Nodal uptake in membranes marked by GFP–Caveolin-1 in the absence and presence of Cripto. (C) Cripto mediates Nodal uptake in membranes expressing GFP–Flotillin-1. (D) Quantification of colocalization of internalized Nodal with GFP–Caveolin-1 and GFP–Flotillin-1 (***P<0.001, Student's _t_-test). (E) Immunogold-labelled anti-Flag antibody internalized by Flag–Nodal in cells transfected with (bottom) or without Cripto (top) decorates uncoated membrane invaginations (arrows). When co-transfected with Cripto, Nodal was also detected at the surface (arrowhead). (F) Immunofluorescence staining of antibody that was internalized by Nodal in transfected COS1 cells (left panel). In cells treated with nystatin (1 h), antibody is not internalized, but instead stains the cell membrane (red arrowheads, right panel). (G) Anti-Cripto western blot of transfected COS1 treated for 4 h with or without nystatin. γ-Tubulin was stained as a loading control. (H) Western blot analysis of whole lysates and conditioned medium of 293T cells that were co-transfected with Cripto and the proteins indicated. Inhibition of palmitoylated proteins by 2-bromo-palmitate (2BP) reduced Nodal secretion and dramatically inhibited precursor processing by endogenous PCs and Flag-tagged Furin (lanes 1, 2, 4 and 5), and to a lesser extent exogenous Flag–PACE4 (lane 6). (I) Incubation of cells with 2-BP inhibits both Nodal signalling in a luciferase reporter assay (left panel) and Nodal-mediated antibody uptake (right panels).
Similar articles
- Extraembryonic proteases regulate Nodal signalling during gastrulation.
Beck S, Le Good JA, Guzman M, Ben Haim N, Roy K, Beermann F, Constam DB. Beck S, et al. Nat Cell Biol. 2002 Dec;4(12):981-5. doi: 10.1038/ncb890. Nat Cell Biol. 2002. PMID: 12447384 - Cripto promotes A-P axis specification independently of its stimulatory effect on Nodal autoinduction.
D'Andrea D, Liguori GL, Le Good JA, Lonardo E, Andersson O, Constam DB, Persico MG, Minchiotti G. D'Andrea D, et al. J Cell Biol. 2008 Feb 11;180(3):597-605. doi: 10.1083/jcb.200709090. J Cell Biol. 2008. PMID: 18268105 Free PMC article. - Cripto localizes Nodal at the limiting membrane of early endosomes.
Blanchet MH, Le Good JA, Oorschot V, Baflast S, Minchiotti G, Klumperman J, Constam DB. Blanchet MH, et al. Sci Signal. 2008 Nov 11;1(45):ra13. doi: 10.1126/scisignal.1165027. Sci Signal. 2008. PMID: 19001664 - Riding shotgun: a dual role for the epidermal growth factor-Cripto/FRL-1/Cryptic protein Cripto in Nodal trafficking.
Constam DB. Constam DB. Traffic. 2009 Jul;10(7):783-91. doi: 10.1111/j.1600-0854.2009.00874.x. Epub 2009 Feb 19. Traffic. 2009. PMID: 19302412 Review. - Role of Cripto-1 in stem cell maintenance and malignant progression.
Bianco C, Rangel MC, Castro NP, Nagaoka T, Rollman K, Gonzales M, Salomon DS. Bianco C, et al. Am J Pathol. 2010 Aug;177(2):532-40. doi: 10.2353/ajpath.2010.100102. Epub 2010 Jul 8. Am J Pathol. 2010. PMID: 20616345 Free PMC article. Review.
Cited by
- Dynamin-Independent Mechanisms of Endocytosis and Receptor Trafficking.
Gundu C, Arruri VK, Yadav P, Navik U, Kumar A, Amalkar VS, Vikram A, Gaddam RR. Gundu C, et al. Cells. 2022 Aug 17;11(16):2557. doi: 10.3390/cells11162557. Cells. 2022. PMID: 36010634 Free PMC article. Review. - Cleavage activates dispatched for Sonic Hedgehog ligand release.
Stewart DP, Marada S, Bodeen WJ, Truong A, Sakurada SM, Pandit T, Pruett-Miller SM, Ogden SK. Stewart DP, et al. Elife. 2018 Jan 23;7:e31678. doi: 10.7554/eLife.31678. Elife. 2018. PMID: 29359685 Free PMC article. - Membrane traffic and synaptic cross-talk during host cell entry by Trypanosoma cruzi.
Butler CE, Tyler KM. Butler CE, et al. Cell Microbiol. 2012 Sep;14(9):1345-53. doi: 10.1111/j.1462-5822.2012.01818.x. Epub 2012 Jul 4. Cell Microbiol. 2012. PMID: 22646288 Free PMC article. - Expression and localization of nodal in bovine oviduct and uterus during different functional stages of oestrus cycle and pregnancy.
Argañaraz ME, Apichela SA, Kenngott R, Vermeheren M, Rodler D, Palma GA, Miceli DC, Sinowatz F. Argañaraz ME, et al. Histochem Cell Biol. 2013 Jan;139(1):89-97. doi: 10.1007/s00418-012-1030-4. Epub 2012 Oct 4. Histochem Cell Biol. 2013. PMID: 23052837 - Targeted enhancement of flotillin-dependent endocytosis augments cellular uptake and impact of cytotoxic drugs.
Fekri F, Abousawan J, Bautista S, Orofiamma L, Dayam RM, Antonescu CN, Karshafian R. Fekri F, et al. Sci Rep. 2019 Nov 28;9(1):17768. doi: 10.1038/s41598-019-54062-9. Sci Rep. 2019. PMID: 31780775 Free PMC article.
References
- Baldwin TA, Ostergaard HL (2002) The protein-tyrosine phosphatase CD45 reaches the cell surface via Golgi-dependent and -independent pathways. J Biol Chem 277: 50333–50340 - PubMed
- Beck S, Le Good JA, Guzman M, Haim NB, Roy K, Beermann F, Constam DB (2002) Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol 4: 981–985 - PubMed
- BenHaim N, Lu C, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Guzman M, Constam DB (2006) The Nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell 11: 1–11 - PubMed
- Bianco C, Kannan S, De Santis M, Seno M, Tang CK, Martinez-Lacaci I, Kim N, Wallace-Jones B, Lippman ME, Ebert AD, Wechselberger C, Salomon DS (1999) Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J Biol Chem 274: 8624–8629 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous