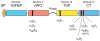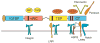Functions and mechanisms of action of CCN matricellular proteins - PubMed (original) (raw)
Review
Functions and mechanisms of action of CCN matricellular proteins
Chih-Chiun Chen et al. Int J Biochem Cell Biol. 2009 Apr.
Abstract
Members of the CCN (CYR61/CTGF/NOV) family have emerged as dynamically expressed, extracellular matrix-associated proteins that play critical roles in cardiovascular and skeletal development, injury repair, fibrotic diseases and cancer. The synthesis of CCN proteins is highly inducible by serum growth factors, cytokines, and environmental stresses such as hypoxia, UV exposure, and mechanical stretch. Consisting of six secreted proteins in vertebrate species, CCNs are typically comprised of four conserved cysteine-rich modular domains. They function primarily through direct binding to specific integrin receptors and heparan sulfate proteoglycans, thereby triggering signal transduction events that culminate in the regulation of cell adhesion, migration, proliferation, gene expression, differentiation, and survival. CCN proteins can also modulate the activities of several growth factors and cytokines, including TGF-beta, TNFalpha, VEGF, BMPs, and Wnt proteins, and may thereby regulate a broad array of biological processes. Recent studies have uncovered novel CCN activities unexpected for matricellular proteins, including their ability to induce apoptosis as cell adhesion substrates, to dictate the cytotoxicity of inflammatory cytokines such as TNFalpha, and to promote hematopoietic stem cell self-renewal. As potent regulators of angiogenesis and chondrogenesis, CCNs are essential for successful cardiovascular and skeletal development during embryogenesis. In the adult, the expression of CCN proteins is associated with injury repair and inflammation, and has been proposed as diagnostic or prognostic markers for diabetic nephropathy, hepatic fibrosis, systemic sclerosis, and several types of cancer. Targeting CCN signaling pathways may hold promise as a strategy of rational therapeutic design.
Figures
Figure 1
Schematics of CCN protein structure and localization of their integrin binding sites. The six CCN proteins include CCN1 (CYR61), CCN2 (CTGF), CCN3 (NOV), CCN4 (WISP-1, ELM1), CCN5 (WISP-2, COP-1), and CCN6 (WISP-3). They share significant structural homology, including an N-terminal secretory signal peptide (SP), followed by modular domains (illustrated in different colors) with sequence homologies to insulin-like growth factor binding protein (IGFBP, module I), von Willebrand factor type C repeat (vWC, module II), thrombospondin type 1 repeat (TSP, module III), and a cysteine knot containing carboxyl domain (CT, module IV). Throughout the four modules are 38 cysteine residues that are highly conserved. CCN5 uniquely lacks the CT domain but conserves domains I–III. A protease-sensitive hinge region with no sequence homology among the CCN proteins separate domains II and III. Specific binding sites (black and hatched bars) for several integrins and HSPGs have been identified for CCN1 and CCN2 (Chen et al., 2000; Leu et al., 2003; Chen et al., 2004a; Leu et al., 2004; Gao and Brigstock, 2004; Gao and Brigstock, 2006).
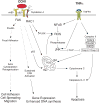
Figure 2
CCN1 signaling and crosstalk with TNFα. Signal transduction initiated by CCN1, a prototypical member of the family, is mediated primarily through binding to α6β1 and syndecan-4 in fibroblasts to support activities including cell adhesion, although αvβ5 is also necessary for fibroblast migration and crosstalk with TNFα (Grzeszkiewicz et al., 2001; Chen et al., 2007). Cell adhesion to CCN1 activates FAK, paxillin, and Rac1, leading to actin cytoskeleton reorganization, cell spreading, and formation of filopodia and lamellipodia (Chen et al., 2001a). Adhesion to CCN1 also induces sustained ERK activation, an activity that is mediated through binding to α6β1-HSPG (Leu et al., 2004). CCN1 induces fibroblast apoptosis by activating p53 and Bax (Todorovic et al., 2005), and converts TNFα from a pro-mitogenic factor into a potent apoptotic molecule through the Rac1-dependent generation of ROS via 5-lipoxygenase and the mitochondria (Chen et al., 2007). CCN1/TNFα-induced apoptosis occurs rapidly (within 4 hours of treatment) without requiring de novo protein synthesis, indicating that CCN1 activates this pathway directly.
Figure 3
Interaction of CCN proteins with other molecules. CCN proteins interact with a variety of cell surface receptors and extracellular ligands, including various integrins, HSPGs, and LRPs (Lau and Lam, 2005; Gao and Brigstock, 2003; Mercurio et al., 2004). Receptors that interact with CCN proteins are shown schematically below the four conserved CCN domains and extracellular proteins that bind CCNs are shown above, aligned with the interacting CCN domains. Whereas CCN3 binds the receptor Notch (Sakamoto et al., 2002), CCN2 has been shown to bind BMPs and TGF-β through the vWC domain and VEGF through the TSP and CT domains (Abreu et al., 2002; Inoki et al., 2002). CCN2 also binds ECM proteins such as fibronectin and perlecan through the CT domain (Nishida et al., 2003; Chen et al., 2004b).
Similar articles
- Role of the CCN protein family in cancer.
Kim H, Son S, Shin I. Kim H, et al. BMB Rep. 2018 Oct;51(10):486-492. doi: 10.5483/BMBRep.2018.51.10.192. BMB Rep. 2018. PMID: 30158025 Free PMC article. - The CCN family proteins in carcinogenesis.
Dhar A, Ray A. Dhar A, et al. Exp Oncol. 2010 Mar;32(1):2-9. Exp Oncol. 2010. PMID: 20332765 Review. - Role of CCN, a vertebrate specific gene family, in development.
Katsube K, Sakamoto K, Tamamura Y, Yamaguchi A. Katsube K, et al. Dev Growth Differ. 2009 Jan;51(1):55-67. doi: 10.1111/j.1440-169X.2009.01077.x. Dev Growth Differ. 2009. PMID: 19128405 Review. - The CCN proteins: important signaling mediators in stem cell differentiation and tumorigenesis.
Zuo GW, Kohls CD, He BC, Chen L, Zhang W, Shi Q, Zhang BQ, Kang Q, Luo J, Luo X, Wagner ER, Kim SH, Restegar F, Haydon RC, Deng ZL, Luu HH, He TC, Luo Q. Zuo GW, et al. Histol Histopathol. 2010 Jun;25(6):795-806. doi: 10.14670/HH-25.795. Histol Histopathol. 2010. PMID: 20376786 Free PMC article. Review. - All in the CCN family: essential matricellular signaling modulators emerge from the bunker.
Leask A, Abraham DJ. Leask A, et al. J Cell Sci. 2006 Dec 1;119(Pt 23):4803-10. doi: 10.1242/jcs.03270. J Cell Sci. 2006. PMID: 17130294 Review.
Cited by
- CCN1 expression in hepatocytes contributes to macrophage infiltration in nonalcoholic fatty liver disease in mice.
Bian Z, Peng Y, You Z, Wang Q, Miao Q, Liu Y, Han X, Qiu D, Li Z, Ma X. Bian Z, et al. J Lipid Res. 2013 Jan;54(1):44-54. doi: 10.1194/jlr.M026013. Epub 2012 Oct 15. J Lipid Res. 2013. PMID: 23071295 Free PMC article. - Molecular profiles of pre- and postoperative breast cancer tumours reveal differentially expressed genes.
Riis ML, Lüders T, Markert EK, Haakensen VD, Nesbakken AJ, Kristensen VN, Bukholm IR. Riis ML, et al. ISRN Oncol. 2012;2012:450267. doi: 10.5402/2012/450267. Epub 2012 Nov 26. ISRN Oncol. 2012. PMID: 23227362 Free PMC article. - Paternal hypercholesterolemia elicits sex-specific exacerbation of atherosclerosis in offspring.
Hernandez R, Li X, Shi J, Dave TR, Zhou T, Chen Q, Zhou C. Hernandez R, et al. JCI Insight. 2024 Sep 10;9(17):e179291. doi: 10.1172/jci.insight.179291. JCI Insight. 2024. PMID: 39253968 Free PMC article. - Restoration of mesenchymal retinal pigmented epithelial cells by TGFβ pathway inhibitors: implications for age-related macular degeneration.
Radeke MJ, Radeke CM, Shih YH, Hu J, Bok D, Johnson LV, Coffey PJ. Radeke MJ, et al. Genome Med. 2015 Jun 19;7(1):58. doi: 10.1186/s13073-015-0183-x. eCollection 2015. Genome Med. 2015. PMID: 26150894 Free PMC article. - CYR61 and TAZ Upregulation and Focal Epithelial to Mesenchymal Transition May Be Early Predictors of Barrett's Esophagus Malignant Progression.
Cardoso J, Mesquita M, Dias Pereira A, Bettencourt-Dias M, Chaves P, Pereira-Leal JB. Cardoso J, et al. PLoS One. 2016 Sep 1;11(9):e0161967. doi: 10.1371/journal.pone.0161967. eCollection 2016. PLoS One. 2016. PMID: 27583562 Free PMC article.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
- Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108–1116. - PubMed
- Aszodi A, Legate KR, Nakchbandi I, Fassler R. What mouse mutants teach us about extracellular matrix function. Annu Rev Cell Dev Biol. 2006;22:591–621. - PubMed
- Athanasopoulos AN, Schneider D, Keiper T, Alt V, Pendurthi UR, Liegibel UM, Sommer U, Nawroth PP, Kasperk C, Chavakis T. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. J Biol Chem. 2007;282:26746–26753. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL081390/HL/NHLBI NIH HHS/United States
- R01 GM078492/GM/NIGMS NIH HHS/United States
- R01 CA046565/CA/NCI NIH HHS/United States
- HL81390/HL/NHLBI NIH HHS/United States
- GM78492/GM/NIGMS NIH HHS/United States
- CA46565/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous