Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation - PubMed (original) (raw)
Effects of cholecystokinin-58 on type 1 cholecystokinin receptor function and regulation
S Vincent Wu et al. Am J Physiol Gastrointest Liver Physiol. 2008 Sep.
Abstract
Cholecystokinin, like many peptide hormones, is present as multiple molecular forms. CCK-58 has been identified as the dominant form in the circulation, whereas most of the studies of CCK-receptor interactions have been performed with CCK-8. Despite both sharing the pharmacophoric region of CCK, representing its carboxy terminal heptapeptide amide, studies in vivo have demonstrated biological diversity of action of the two peptides, with CCK-58, but not CCK-8, stimulating pancreatic fluid secretion and lengthening the interval between meals. Here, we have directly studied the ability of these two CCK peptides to bind to the type 1 CCK receptor and to stimulate it to elicit an intracellular calcium response. The calcium response relative to receptor occupation was identical for CCK-58 and CCK-8, with the longer peptide binding with approximately fivefold lower affinity. We also examined the ability of the two peptides to elicit receptor internalization using morphological techniques and to disrupt the constitutive oligomerization of the CCK receptor using receptor bioluminescence resonance energy transfer. Here, both full agonist peptides had similar effects on these regulatory processes. These data suggest that both molecular forms of CCK act at the CCK1 receptor quite similarly and elicit similar regulatory processes for that receptor, suggesting that the differences in biological activity observed in vivo most likely reflect differences in the clearance and/or metabolism of these long and short forms of CCK peptides.
Figures
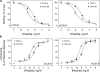
Fig. 1.
CCK-58 and CCK-8 receptor binding and biological activity. Shown are competition-binding curves (A) and stimulated intracellular calcium response (Δ[Calcium]i) curves (B) to CCK-8 and CCK-58 in rat and human CCK1 receptor-bearing cells. All values represent the means ± SE of data from at least 3 independent experiments (for binding) and duplicate determinations (for calcium). rCCK1R and hCCK1R, rat and human type 1 CCK receptors, respectively; Max, maximum; brackets denote concentration.
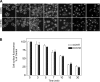
Fig. 2.
Agonist-induced CCK receptor internalization. A: representative confocal microscopic images demonstrating internalization of yellow fluorescent protein (YFP)-tagged CCK1 receptors expressed on Chinese hamster ovary (CHO) cells after their occupation with nonfluorescent CCK peptides. Time points after stimulation are noted. Like CCK-8, CCK-58 stimulated prompt and extensive CCK receptor internalization, with similar patterns of distribution. The kinetics of internalization were also similar after stimulation with the 2 CCK peptides. Scale bar = 25 μm. B: quantitation of the receptor on the cell surface under these conditions. Results reflect means ± SE of data from 3 independent experiments.
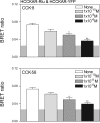
Fig. 3.
CCK-58 and CCK-8 disrupt CCK1 receptor oligomerization. Shown are bioluminescence resonance energy transfer (BRET) ratios obtained from COS cells coexpressing Renilla luciferase (Rlu)-and YFP-tagged human CCK1 receptors (HCCKAR). Cells expressing the CCK receptor constructs were incubated with increasing concentrations of either CCK-8 or CCK-58 prior to measurement of the BRET signals. Concentration-dependent decreases in the BRET signals were observed after stimulation with each of the peptides. The shaded area represents the background BRET ratio that can be measured upon expression of structurally unrelated receptors on the cell surface or upon expression of a donor-acceptor pair with one in the plasma membrane and the other in the cytosol. Signals above this are considered to be significant. Data represent means ± SE of 4 independent experiments. *P < 0.05, **P < 0.01 compared with BRET signals obtained without exposure to CCK peptides.
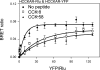
Fig. 4.
Saturation BRET analysis. Shown are BRET saturation curves plotted as the ratios of YFP fluorescence to Rlu luminescence obtained when COS cells coexpressing fixed concentrations of Rlu-tagged CCK1 receptors (donor) and increasing the concentrations of YFP-tagged CCK1 receptors (acceptor) were exposed to the noted peptides. For control cells not exposed to CCK, the BRET ratios generated exponential curves that increased until values reached saturation, as reflected in an asymptote. In contrast, CCK-8 and CCK-58 stimulation yielded BRET saturation curves that were not statistically different from linear fits. Data are represented as means ± SE of 4 independent experiments.
Similar articles
- Identification of two amino acids of the human cholecystokinin-A receptor that interact with the N-terminal moiety of cholecystokinin.
Kennedy K, Gigoux V, Escrieut C, Maigret B, Martinez J, Moroder L, Fréhel D, Gully D, Vaysse N, Fourmy D. Kennedy K, et al. J Biol Chem. 1997 Jan 31;272(5):2920-6. doi: 10.1074/jbc.272.5.2920. J Biol Chem. 1997. PMID: 9006937 - Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A.
Tarasova NI, Stauber RH, Choi JK, Hudson EA, Czerwinski G, Miller JL, Pavlakis GN, Michejda CJ, Wank SA. Tarasova NI, et al. J Biol Chem. 1997 Jun 6;272(23):14817-24. doi: 10.1074/jbc.272.23.14817. J Biol Chem. 1997. PMID: 9169450 - Natural and synthetic CCK-58. Novel reagents for studying cholecystokinin physiology.
Reeve JR Jr, Eysselein VE, Ho FJ, Chew P, Vigna SR, Liddle RA, Evans C. Reeve JR Jr, et al. Ann N Y Acad Sci. 1994 Mar 23;713:11-21. doi: 10.1111/j.1749-6632.1994.tb44047.x. Ann N Y Acad Sci. 1994. PMID: 7514372 Review. - Therapeutic potential for novel drugs targeting the type 1 cholecystokinin receptor.
Cawston EE, Miller LJ. Cawston EE, et al. Br J Pharmacol. 2010 Mar;159(5):1009-21. doi: 10.1111/j.1476-5381.2009.00489.x. Epub 2009 Nov 18. Br J Pharmacol. 2010. PMID: 19922535 Free PMC article. Review.
Cited by
- Metabolic Actions of the Type 1 Cholecystokinin Receptor: Its Potential as a Therapeutic Target.
Miller LJ, Desai AJ. Miller LJ, et al. Trends Endocrinol Metab. 2016 Sep;27(9):609-619. doi: 10.1016/j.tem.2016.04.002. Epub 2016 May 4. Trends Endocrinol Metab. 2016. PMID: 27156041 Free PMC article. Review. - Cholecystokinin-33 acutely attenuates food foraging, hoarding and intake in Siberian hamsters.
Teubner BJ, Bartness TJ. Teubner BJ, et al. Peptides. 2010 Apr;31(4):618-24. doi: 10.1016/j.peptides.2009.12.010. Epub 2009 Dec 16. Peptides. 2010. PMID: 20025915 Free PMC article. - Cholecystokinin-58 and cholecystokinin-8 exhibit similar actions on calcium signaling, zymogen secretion, and cell fate in murine pancreatic acinar cells.
Criddle DN, Booth DM, Mukherjee R, McLaughlin E, Green GM, Sutton R, Petersen OH, Reeve JR Jr. Criddle DN, et al. Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297(6):G1085-92. doi: 10.1152/ajpgi.00119.2009. Epub 2009 Oct 8. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19815626 Free PMC article. - Cholecystokinin-A signaling regulates automaticity of pacemaker cardiomyocytes.
Ruan H, Mandla R, Ravi N, Galang G, Soe AW, Olgin JE, Lang D, Vedantham V. Ruan H, et al. Front Physiol. 2023 Dec 21;14:1284673. doi: 10.3389/fphys.2023.1284673. eCollection 2023. Front Physiol. 2023. PMID: 38179138 Free PMC article. - TMEM16B determines cholecystokinin sensitivity of intestinal vagal afferents of nodose neurons.
Wang R, Lu Y, Cicha MZ, Singh MV, Benson CJ, Madden CJ, Chapleau MW, Abboud FM. Wang R, et al. JCI Insight. 2019 Mar 7;4(5):e122058. doi: 10.1172/jci.insight.122058. eCollection 2019 Mar 7. JCI Insight. 2019. PMID: 30843875 Free PMC article.
References
- Angers S, Salahpour A, Bouvier M. Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435, 2002. - PubMed
- Burton-Freeman B, Gietzen DW, Schneeman BO. Cholecystokinin and serotonin receptors in the regulation of fat-induced satiety in rats. Am J Physiol Regul Integr Comp Physiol 276: R429–R434, 1999. - PubMed
- Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278: 52972–52979, 2003. - PubMed
- Cheng ZJ, Miller LJ. Agonist-dependent dissociation of oligomeric complexes of G protein-coupled cholecystokinin receptors demonstrated in living cells using bioluminescence resonance energy transfer. J Biol Chem 276: 48040–48047, 2001. - PubMed
- Dong M, Ding XQ, Thomas SE, Gao F, Lam PC, Abagyan R, Miller LJ. Role of lysine187 within the second extracellular loop of the type A cholecystokinin receptor in agonist-induced activation. Use of complementary charge-reversal mutagenesis to define a functionally important interdomain interaction. Biochemistry 46: 4522–4531, 2007. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK-41301/DK/NIDDK NIH HHS/United States
- DK-33850/DK/NIDDK NIH HHS/United States
- R01 DK033850/DK/NIDDK NIH HHS/United States
- DK-32878/DK/NIDDK NIH HHS/United States
- P50-AA011999/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases