A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance - PubMed (original) (raw)
. 2008 Oct;10(10):1146-53.
doi: 10.1038/ncb1774. Epub 2008 Sep 7.
Helge Gad, Stella Y Lee, Alexander Mironov, Leiliang Zhang, Galina V Beznoussenko, Carmen Valente, Gabriele Turacchio, Akua N Bonsra, Guangwei Du, Gianluca Baldanzi, Andrea Graziani, Sylvain Bourgoin, Michael A Frohman, Alberto Luini, Victor W Hsu
Affiliations
- PMID: 18776900
- PMCID: PMC2756218
- DOI: 10.1038/ncb1774
A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance
Jia-Shu Yang et al. Nat Cell Biol. 2008 Oct.
Abstract
Proteins essential for vesicle formation by the Coat Protein I (COPI) complex are being identified, but less is known about the role of specific lipids. Brefeldin-A ADP-ribosylated substrate (BARS) functions in the fission step of COPI vesicle formation. Here, we show that BARS induces membrane curvature in cooperation with phosphatidic acid. This finding has allowed us to further delineate COPI vesicle fission into two sub-stages: 1) an earlier stage of bud-neck constriction, in which BARS and other COPI components are required, and 2) a later stage of bud-neck scission, in which phosphatidic acid generated by phospholipase D2 (PLD2) is also required. Moreover, in contrast to the disruption of the Golgi seen on perturbing the core COPI components (such as coatomer), inhibition of PLD2 causes milder disruptions, suggesting that such COPI components have additional roles in maintaining Golgi structure other than through COPI vesicle formation.
Figures
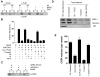
Figure 1. Liposome tubulation by BARS requires PA
(A) Binding by BARS to liposomes with increasing level of additional specified lipid. (B) EM examination of liposomes bound by BARS; bar, 200 nm. The fraction that exhibited tubulation was quantified, with mean and standard error from three experiments shown. (C) Quantitation for the ability of other proteins to induce liposome tubulation, as analyzed by EM.
Figure 2. Perturbation of PA generated by PLD2 affects COPI transport
(A) Gel shows knockdown of proteins (upper panels) with loading controls (lower panels). Graph shows quantitation of retrograde transport of the chimeric KDELR, with the mean from three experiments and standard error shown. (B) Gel shows knockdown of proteins. Graph again shows quantitation of retrograde transport of the chimeric KDELR. (C) Quantitation of retrograde transport of the chimeric KDELR upon microinjection of different antibodies in conditions, with the mean from three experiments and standard error shown. (D) First stage shows ARF1-dependent recruitment of coatomer to Golgi membrane (above). Second stage shows COPI vesicle formation, as reflected by the release of coatomer from Golgi membrane (below).
Figure 3. COPI vesicle formation requires PA generated by PLD2
(A) Vesicle formation as reflected by the release of β–COP from Golgi membrane at the second stage. (B) Quantitation of vesicle formation as reflected by the release of β–COP from Golgi membrane at the second stage. The mean from three experiments with standard error is shown. (C) Vesicle formation as reflected by the release of β–COP from Golgi membrane at the second stage. (D) Cargo sorting into COPI vesicles, as reflected by the release of cargo proteins from Golgi membrane at the second stage. (E) Vesicle formation assessed by immunogold EM. The mean from three experiments with standard error is shown.
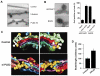
Figure 4. PLD2 is required for at a late stage of COPI vesicle fission
(A) EM examination of Golgi membrane after the second stage; bar, 50 nm. As comparison, Golgi membrane prior to the incubation is shown as control. (B) The distributions of proteins on Golgi membrane after the second stage as assessed by immunogold EM; bar, 50 nm. Quantitation with the mean from three experiments and standard error is also shown. (C) Three-dimensional reconstruction of a representative Golgi from either control or PLD2-depleted cells is shown in two opposite views. Yellow highlights buds. (D) Quantitation of buds accumulation. The mean and standard deviation, derived from analyzing ten randomly selected Golgi profiles in each condition, are shown.
Figure 5. Golgi morphology and COPI distribution upon PLD2 depletion
(A) Relative distributions of COPI labeling at the Golgi. The mean from ten randomly selected Golgi profiles with standard deviation is shown. (B) Golgi in control cells. (C-E) Golgi in PLD2-depleted cells; bar, 200 nm.
Similar articles
- The late stage of COPI vesicle fission requires shorter forms of phosphatidic acid and diacylglycerol.
Park SY, Yang JS, Li Z, Deng P, Zhu X, Young D, Ericsson M, Andringa RLH, Minnaard AJ, Zhu C, Sun F, Moody DB, Morris AJ, Fan J, Hsu VW. Park SY, et al. Nat Commun. 2019 Jul 30;10(1):3409. doi: 10.1038/s41467-019-11324-4. Nat Commun. 2019. PMID: 31363100 Free PMC article. - A role for BARS at the fission step of COPI vesicle formation from Golgi membrane.
Yang JS, Lee SY, Spanò S, Gad H, Zhang L, Nie Z, Bonazzi M, Corda D, Luini A, Hsu VW. Yang JS, et al. EMBO J. 2005 Dec 7;24(23):4133-43. doi: 10.1038/sj.emboj.7600873. Epub 2005 Nov 17. EMBO J. 2005. PMID: 16292346 Free PMC article. - Diacylglycerol is required for the formation of COPI vesicles in the Golgi-to-ER transport pathway.
Fernández-Ulibarri I, Vilella M, Lázaro-Diéguez F, Sarri E, Martínez SE, Jiménez N, Claro E, Mérida I, Burger KN, Egea G. Fernández-Ulibarri I, et al. Mol Biol Cell. 2007 Sep;18(9):3250-63. doi: 10.1091/mbc.e07-04-0334. Epub 2007 Jun 13. Mol Biol Cell. 2007. PMID: 17567948 Free PMC article. - The structure of COPI vesicles and regulation of vesicle turnover.
Taylor RJ, Tagiltsev G, Briggs JAG. Taylor RJ, et al. FEBS Lett. 2023 Mar;597(6):819-835. doi: 10.1002/1873-3468.14560. Epub 2022 Dec 30. FEBS Lett. 2023. PMID: 36513395 Review. - Components of the CtBP1/BARS-dependent fission machinery.
Valente C, Luini A, Corda D. Valente C, et al. Histochem Cell Biol. 2013 Oct;140(4):407-21. doi: 10.1007/s00418-013-1138-1. Epub 2013 Sep 1. Histochem Cell Biol. 2013. PMID: 23996193 Review.
Cited by
- Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy.
Cui L, Li H, Xi Y, Hu Q, Liu H, Fan J, Xiang Y, Zhang X, Shui W, Lai Y. Cui L, et al. Mol Biomed. 2022 Sep 21;3(1):29. doi: 10.1186/s43556-022-00090-3. Mol Biomed. 2022. PMID: 36129576 Free PMC article. Review. - Mechanisms of COPI vesicle formation.
Hsu VW, Yang JS. Hsu VW, et al. FEBS Lett. 2009 Dec 3;583(23):3758-63. doi: 10.1016/j.febslet.2009.10.056. Epub 2009 Oct 23. FEBS Lett. 2009. PMID: 19854177 Free PMC article. Review. - A WDR35-dependent coat protein complex transports ciliary membrane cargo vesicles to cilia.
Quidwai T, Wang J, Hall EA, Petriman NA, Leng W, Kiesel P, Wells JN, Murphy LC, Keighren MA, Marsh JA, Lorentzen E, Pigino G, Mill P. Quidwai T, et al. Elife. 2021 Nov 4;10:e69786. doi: 10.7554/eLife.69786. Elife. 2021. PMID: 34734804 Free PMC article. - The synaptic ribbon is a site of phosphatidic acid generation in ribbon synapses.
Schwarz K, Natarajan S, Kassas N, Vitale N, Schmitz F. Schwarz K, et al. J Neurosci. 2011 Nov 2;31(44):15996-6011. doi: 10.1523/JNEUROSCI.2965-11.2011. J Neurosci. 2011. PMID: 22049442 Free PMC article. - Mitochondria: signaling with phosphatidic acid.
Yang CY, Frohman MA. Yang CY, et al. Int J Biochem Cell Biol. 2012 Aug;44(8):1346-50. doi: 10.1016/j.biocel.2012.05.006. Epub 2012 May 15. Int J Biochem Cell Biol. 2012. PMID: 22609101 Free PMC article. Review.
References
- Lee MC, Miller EA, Goldberg J, Orci L, Schekman R. Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol. 2004;20:87–123. - PubMed
- Rabouille C, Klumperman J. Opinion: The maturing role of COPI vesicles in intra-Golgi transport. Nat Rev Mol Cell Biol. 2005;6:812–7. - PubMed
- Nie Z, Hirsch DS, Randazzo PA. Arf and its many interactors. Curr Opin Cell Biol. 2003;15:396–404. - PubMed
- D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. - PubMed
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM058615/GM/NIGMS NIH HHS/United States
- R01 GM058615/GM/NIGMS NIH HHS/United States
- GM071475/GM/NIGMS NIH HHS/United States
- R01 GM071520/GM/NIGMS NIH HHS/United States
- R01 GM058615-07/GM/NIGMS NIH HHS/United States
- GGP04235/TI_/Telethon/Italy
- GM071520/GM/NIGMS NIH HHS/United States
- R37 GM058615/GM/NIGMS NIH HHS/United States
- R01 GM071475/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources