Multiple changes in sialic acid biology during human evolution - PubMed (original) (raw)
Review
Multiple changes in sialic acid biology during human evolution
Ajit Varki. Glycoconj J. 2009 Apr.
Abstract
Humans are genetically very similar to "great apes", (chimpanzees, bonobos, gorillas and orangutans), our closest evolutionary relatives. We have discovered multiple genetic and biochemical differences between humans and these other hominids, in relation to sialic acids and in Siglecs (Sia-recognizing Ig superfamily lectins). An inactivating mutation in the CMAH gene eliminated human expression of N-glycolylneuraminic acid (Neu5Gc) a major sialic acid in "great apes". Additional human-specific changes have been found, affecting at least 10 of the <60 genes known to be involved in the biology of sialic acids. There are potential implications for unique features of humans, as well as for human susceptibility or resistance to disease. Additionally, metabolic incorporation of Neu5Gc from animal-derived materials occurs into biotherapeutic molecules and cellular preparations--and into human tissues from dietary sources, particularly red meat and milk products. As humans also have varying and sometime high levels of circulating anti-Neu5Gc antibodies, there are implications for biotechnology products, and for some human diseases associated with chronic inflammation.
Figures
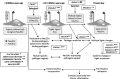
Fig. 1
Suggested scenario for multiple changes in sialic acid biology during human evolution. The initial loss of Neu5Gc expression during human evolution could have occurred randomly, or due to selection by a pathogen that preferentially recognized Neu5Gc on cell surfaces (e.g., a form of hominid malaria, or a bacterial toxin). Regardless of the reason, the resultant loss of Neu5Gc-binding sites for some CD33rSiglecs should have generated unusual immune activation, and further selection was likely required to allow adjustment for binding of Neu5Ac in some Siglecs, with elimination or loss of binding by others. Thus, all the other human specific changes in sialic acid biology discussed in the text could have resulted from adjustments to the original event of CMAH inactivation. Of course, other scenarios are possible. A non-genetic complexity is also indicated, in which dietary Neu5Gc can accumulate in human tissues in the face of an anti-Neu5Gc response, potentially facilitating diseases associated with chronic inflammation. (Modified from Varki A. Nature 446: 1023, 2007). Note that while CD33rSiglecs are shown as binding to sialic acids on the same cell surface, they can also potentially detect high densities of sialic acids on other cell surfaces or on Neu5Ac-expressing pathogens (which are common in humans)
Similar articles
- Colloquium paper: uniquely human evolution of sialic acid genetics and biology.
Varki A. Varki A. Proc Natl Acad Sci U S A. 2010 May 11;107 Suppl 2(Suppl 2):8939-46. doi: 10.1073/pnas.0914634107. Epub 2010 May 5. Proc Natl Acad Sci U S A. 2010. PMID: 20445087 Free PMC article. - Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs.
Brinkman-Van der Linden EC, Sjoberg ER, Juneja LR, Crocker PR, Varki N, Varki A. Brinkman-Van der Linden EC, et al. J Biol Chem. 2000 Mar 24;275(12):8633-40. doi: 10.1074/jbc.275.12.8633. J Biol Chem. 2000. PMID: 10722703 - Loss of N-glycolylneuraminic acid in humans: Mechanisms, consequences, and implications for hominid evolution.
Varki A. Varki A. Am J Phys Anthropol. 2001;Suppl 33(Suppl ):54-69. doi: 10.1002/ajpa.10018.abs. Am J Phys Anthropol. 2001. PMID: 11786991 Free PMC article. Review. - Possible Influences of Endogenous and Exogenous Ligands on the Evolution of Human Siglecs.
Angata T. Angata T. Front Immunol. 2018 Dec 4;9:2885. doi: 10.3389/fimmu.2018.02885. eCollection 2018. Front Immunol. 2018. PMID: 30564250 Free PMC article. Review. - A second uniquely human mutation affecting sialic acid biology.
Angata T, Varki NM, Varki A. Angata T, et al. J Biol Chem. 2001 Oct 26;276(43):40282-7. doi: 10.1074/jbc.M105926200. Epub 2001 Aug 23. J Biol Chem. 2001. PMID: 11546777
Cited by
- Functions and Biosynthesis of O-Acetylated Sialic Acids.
Mandal C, Schwartz-Albiez R, Vlasak R. Mandal C, et al. Top Curr Chem. 2015;366:1-30. doi: 10.1007/128_2011_310. Top Curr Chem. 2015. PMID: 22371169 Free PMC article. Review. - Preclinical Evaluation of a Near-Infrared Labelled Antibody Targeting the Tumour Associated Xenoantigen N-Glycolyl-Neuraminic Acid GM3 Ganglioside.
Barreto K, Bernhard W, Toledo D, Jett K, Casaco A, León K, Geyer CR. Barreto K, et al. Mol Imaging Biol. 2025 Jun 17. doi: 10.1007/s11307-025-02026-z. Online ahead of print. Mol Imaging Biol. 2025. PMID: 40526182 - A systematic approach to protein glycosylation analysis: a path through the maze.
Mariño K, Bones J, Kattla JJ, Rudd PM. Mariño K, et al. Nat Chem Biol. 2010 Oct;6(10):713-23. doi: 10.1038/nchembio.437. Epub 2010 Sep 17. Nat Chem Biol. 2010. PMID: 20852609 Review. - The _N-_Glycosylation of Mouse Immunoglobulin G (IgG)-Fragment Crystallizable Differs Between IgG Subclasses and Strains.
de Haan N, Reiding KR, Krištić J, Hipgrave Ederveen AL, Lauc G, Wuhrer M. de Haan N, et al. Front Immunol. 2017 May 31;8:608. doi: 10.3389/fimmu.2017.00608. eCollection 2017. Front Immunol. 2017. PMID: 28620376 Free PMC article. - Mammalian cell ganglioside-binding specificities of E. coli enterotoxins LT-IIb and variant LT-IIb(T13I).
Berenson CS, Nawar HF, Yohe HC, Castle SA, Ashline DJ, Reinhold VN, Hajishengallis G, Connell TD. Berenson CS, et al. Glycobiology. 2010 Jan;20(1):41-54. doi: 10.1093/glycob/cwp141. Epub 2009 Sep 12. Glycobiology. 2010. PMID: 19749203 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials