Role of mucosal dendritic cells in inflammatory bowel disease - PubMed (original) (raw)
Review
Role of mucosal dendritic cells in inflammatory bowel disease
Jan Hendrik Niess. World J Gastroenterol. 2008.
Abstract
The gastrointestinal innate and adaptive immune system continuously faces the challenge of potent stimuli from the commensal microflora and food constituents. These local immune responses require a tight control, the outcome of which is in most cases the induction of tolerance. Local T cell immunity is an important compartment of the specific intestinal immune system. T cell reactivity is programmed during the initial stage of its activation by professional presenting cells. Mucosal dendritic cells (DCs) are assumed to play key roles in regulating immune responses in the antigen-rich gastrointestinal environment. Mucosal DCs are a heterogeneous population that can either initiate (innate and adaptive) immune responses, or control intestinal inflammation and maintain tolerance. Defects in this regulation are supposed to lead to the two major forms of inflammatory bowel disease (IBD), Crohn's disease (CD) and ulcerative colitis (UC). This review will discuss the emerging role of mucosal DCs in regulating intestinal inflammation and immune responses.
Figures
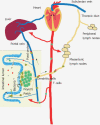
Figure 1
Bacterial and food antigens are continuously surveyed by the mucosal immune system, processed and transported via the lymph to mesenteric lymph nodes or via the portal vein to the liver. DCs present processed luminal antigens to naïve T cells to achieve tolerance or to initiate host defence to pathogens.
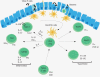
Figure 2
DCs continuously survey the intestinal lumen, phagocytose and process luminal antigens and present them to T cells, which will differentiate in presence of IL-6 and TGF-β to IL-17A and IL-17F producing TH17 cells, in presence of IL-12 and IFN-γ into TH1 cells or in presence of IL-4 into TH2 cells. nTreg, natural occurring regulatory T cell; iTreg, inducible regulatory T cells; Tr1, regulating T cell.
Similar articles
- Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease.
Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM. Saez A, et al. Int J Mol Sci. 2021 Jul 16;22(14):7618. doi: 10.3390/ijms22147618. Int J Mol Sci. 2021. PMID: 34299236 Free PMC article. Review. - Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease.
Xu XR, Liu CQ, Feng BS, Liu ZJ. Xu XR, et al. World J Gastroenterol. 2014 Mar 28;20(12):3255-64. doi: 10.3748/wjg.v20.i12.3255. World J Gastroenterol. 2014. PMID: 24695798 Free PMC article. Review. - Emerging Role of Dendritic Cell Intervention in the Treatment of Inflammatory Bowel Disease.
Sun D, Li C, Chen S, Zhang X. Sun D, et al. Biomed Res Int. 2022 Oct 10;2022:7025634. doi: 10.1155/2022/7025634. eCollection 2022. Biomed Res Int. 2022. PMID: 36262975 Free PMC article. Review. - CD40 and CD86 upregulation with divergent CMRF44 expression on blood dendritic cells in inflammatory bowel diseases.
Vuckovic S, Florin TH, Khalil D, Zhang MF, Patel K, Hamilton I, Hart DN. Vuckovic S, et al. Am J Gastroenterol. 2001 Oct;96(10):2946-56. doi: 10.1111/j.1572-0241.2001.04686.x. Am J Gastroenterol. 2001. PMID: 11693331
Cited by
- Enterocyte dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin expression in inflammatory bowel disease.
Zeng JQ, Xu CD, Zhou T, Wu J, Lin K, Liu W, Wang XQ. Zeng JQ, et al. World J Gastroenterol. 2015 Jan 7;21(1):187-95. doi: 10.3748/wjg.v21.i1.187. World J Gastroenterol. 2015. PMID: 25574091 Free PMC article. - Leveraging Organ-on-Chip Models to Investigate Host-Microbiota Dynamics and Targeted Therapies for Inflammatory Bowel Disease.
Kaden T, Alonso-Román R, Stallhofer J, Gresnigt MS, Hube B, Mosig AS. Kaden T, et al. Adv Healthc Mater. 2025 Apr;14(10):e2402756. doi: 10.1002/adhm.202402756. Epub 2024 Nov 3. Adv Healthc Mater. 2025. PMID: 39491534 Free PMC article. Review. - Gastrointestinal microbiota and some children diseases: a review.
Weber TK, Polanco I. Weber TK, et al. Gastroenterol Res Pract. 2012;2012:676585. doi: 10.1155/2012/676585. Epub 2012 Oct 30. Gastroenterol Res Pract. 2012. PMID: 23197978 Free PMC article. - Gut microbiota in various childhood disorders: Implication and indications.
Saeed NK, Al-Beltagi M, Bediwy AS, El-Sawaf Y, Toema O. Saeed NK, et al. World J Gastroenterol. 2022 May 14;28(18):1875-1901. doi: 10.3748/wjg.v28.i18.1875. World J Gastroenterol. 2022. PMID: 35664966 Free PMC article. Review. - Oral vitamin D3 supplementation reduces monocyte-derived dendritic cell maturation and cytokine production in Crohn's disease patients.
Bartels LE, Bendix M, Hvas CL, Jørgensen SP, Agnholt J, Agger R, Dahlerup JF. Bartels LE, et al. Inflammopharmacology. 2014 Apr;22(2):95-103. doi: 10.1007/s10787-013-0197-1. Epub 2013 Dec 29. Inflammopharmacology. 2014. PMID: 24374976 Clinical Trial.
References
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
- Strober W. Immunology. Unraveling gut inflammation. Science. 2006;313:1052–1054. - PubMed
- Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol. 2001;1:59–67. - PubMed
- Niess JH, Reinecker HC. Dendritic cells in the recognition of intestinal microbiota. Cell Microbiol. 2006;8:558–564. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources