GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival - PubMed (original) (raw)
GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival
Ivo Sonderegger et al. J Exp Med. 2008.
Abstract
Granulocyte macrophage-colony stimulating factor (GM-CSF) is critically involved in development of organ-related autoimmune inflammatory diseases including experimental allergic encephalitis and collagen-induced arthritis. Roles of GM-CSF in the initiation and in the effector phase of the autoimmune response have been proposed. Our study was designed to investigate the mechanisms of GM-CSF in autoimmunity using a model of autoimmune heart inflammatory disease (myocarditis). The pathological sequel after immunization with heart myosin has been shown previously to depend on IL-1, IL-6, IL-23, and IL-17. We found that innate GM-CSF was critical for IL-6 and IL-23 responses by dendritic cells and generation of pathological Th17 cells in vivo. Moreover, GM-CSF promoted autoimmunity by enhancing IL-6-dependent survival of antigen specific CD4(+) T cells. These results suggest a novel role for GM-CSF in promoting generation and maintenance of Th17 cells by regulation of IL-6 and IL-23 in vivo.
Figures
Figure 1.
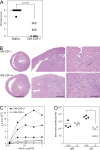
Reduced autoimmune myocarditis, T cell proliferation, and antibody responses to heart myosin in GM-CSF–deficient mice. GM-CSF−/− (open circles) and WT (closed circles) mice were immunized at days 0 and 7 with myhcα614-629 peptide in CFA. Hearts were removed at day 21 and fixed in 4% formalin. (A) The severity of inflammatory infiltrates was scored as described in Materials and methods. (B) Representative images of Hematoxylin and Eosin stainings of WT and GM-CSF−/− heart sections. Bars: (left) 2 mm; (middle) 0.5 mm; (right) 0.1 mm. (C) CD4+ T cells were isolated from the spleen 21 d after immunization and in vitro restimulated with BM-DC and titrating amounts of myhcα614-629. Proliferation was assessed by 3H-thymidine incorporation after 3 d of culture. (D) Serum titers of myhcα614-629-specific IgM and IgG at day 21 d after immunization measured by ELISA. Horizontal lines represent the mean titer for each group (D) and the median histological scores of groups (A). Data shown are representative of three to four experiments. *, P < 0.05.
Figure 2.
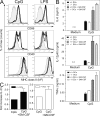
GM-CSF enhances TLR-induced production of proinflammatory cytokines by DC. DC isolated from spleens of naive WT and GM-CSF−/− mice were stimulated with 1 μM CpG or 1 μg/ml LPS in vitro. (A) Surface expression of CD86, CD40, and MHC class II was assessed by flow cytometry after 6 h. Unstimulated cells (shaded curve), stimulated WT (solid line), GM-CSF−/− (dashed line), and GM-CSF−/− DC in the presence of 20 ng/ml rGM-CSF (dotted line). (B) ELISA measurement of IL-6, IL-12(p40/p70), and TNF-α in supernatants of DC stimulated for 24 h with CpG in the presence and absence of 20 ng/ml GM-CSF. (C) IL-23p19 measured by quantitative PCR of DC stimulated for 4 h with 1 μM CpG in the presence and absence of GM-CSF. One representative experiment of at least two is shown. Error bars indicate SD.
Figure 3.
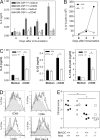
Reduced production of IL-6 in immunized GM-CSF−/− mice. WT and GM-CSF−/− mice were immunized s.c. with myhcα614-629/CFA as described in the Fig. 1 legend. (A) At the times indicated, CD11b+ and CD11c+ cells were isolated by magnetic sorting and cultured in the presence and absence of anti-CD40 for 20 h. IL-6 concentrations in the supernatant was measured by ELISA. (B) Expression of IL-6 relative to β-actin was assessed by quantitative PCR using cDNA generated from CD11b+ cells. (C) At day 7, CD11c+ cells were isolated by magnetic sorting and cultured in the presence and absence of anti-CD40 for 24 h (4 × 105 DC per well). IL-6, IL-23, and IL-12p40 levels in the supernatant were measured by ELISA. (D) Analysis of draining LN cells by flow cytometry at day 4 after immunization. Histograms show expression of CD80, CD86, CD40, and MHC class II gated on CD11c+ cells. Continuous and dotted lines represent cells of immunized WT and GM-CSF−/− mice, respectively. Shaded curves represent cells of naive mice. (E) BM-derived DC of WT and GM-CSF−/− mice were pulsed with 10 μg/ml myhcα614-629 and stimulated with 1 μg/ml LPS and 5 μg/ml anti-CD40 for 4 h before injection into WT and GM-CSF−/− mice at days 0, 2, and 4. At day 10, hearts were removed and myocarditis scored by histology. Horizontal lines indicate median. Error bars in A indicate SD of triplicate culture wells. Cells were isolated from pooled draining LN of three mice per time point. Error bars in C indicate SD of triplicate cultures of draining LN DC of four to five mice per time point. Data shown are representative of at least two experiments. *, P < 0.05.
Figure 4.
GM-CSF production by CD4+ T cells promotes proliferation and differentiation of Th17, but not Th1 or Th2, cells in vitro. Naive OVA323-339 TCR transgenic CD4+ T cells purified from spleens of DO11.10/GM-CSF−/− or DO11.10/GM-CSF+/+ mice were cocultured with splenic DC purified from GM-CSF-deficient (KO) or WT mice in the presence of indicated concentrations of ovalbumin protein or OVA323-339 peptide. (A) CD4+ T cell activation measured by CD25 and CD62L surface expression after 3 d of culture. (B) CD4 T cell proliferation measured by pulsing cells with 3H-Thymidine for the last 12 h of a 60-h coculture. (C) Th1/Th2 cell differentiation driven by indicated antigen concentrations was determined by intracellular staining of IFN-γ and IL-4 after 5 d of coculture. (D and E) Cocultures were stimulated by 10 μM OVA323-339 and 1 μM CpG for 4 d. IL-17, IFN-γ, and GM-CSF production by CD4+ T cells was determined by intracellular staining in cultures containing GM-CSF+/+ CD4+ T cells and DC (D) or indicated combinations of WT and KO T cells and DC (E). (F) WT and KO CD4+ T cells and DC were cultured with anti-CD3 in Th17-polarizing conditions including 5 ng/ml TGFβ1, 20 ng/ml IL-6, 20 ng/ml IL-21, 1 μM CpG, and 100 μg/ml curdlan. Cytokine production was determined by intracellular staining at day 4.
Figure 5.
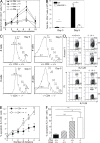
GM-CSF prevents apoptosis of specific T cells. CD4+ T cells were purified from DO11.10/GM-CSF+/+ and DO11.10/GM-CSF−/−mice and labeled with CFSE before intravenous injection into WT or GM-CSF−/− mice (5 × 106 cells per mouse) at day 3. Mice were immunized s.c. with 200 μg OVA323-339 peptide in CFA at day 0. (A) KJ1-26+ CD4 T cell expansion was monitored over 10 d in the blood of mice (n = 4). The frequency of KJ1-26+ CD4+ T cells in control animals immunized with CFA only was <0.5%. (B) Total number of KJ1-26+ cells in draining LN at days 3 and 5 after immunization. Filled columns, DO11.10/GM-CSF+/+CD4+→GM-CSF+/+ mice; open columns, DO11.10/GM-CSF−/− CD4+→GM-CSF−/− mice (n = 3). (C) Proliferation of KJ1-26+ CD4+ T cells is shown by CFSE dilution at days 3 (left) and 5 (right). (D) Annexin V (top) and PI (bottom) staining of KJ1-26+ CD4+ T cells in draining LN at days 3 and day 5. (E) Shown is the number of cell division determined by CFSE dilution and percentage of apoptotic (Annexin V+ PI−) KJ1-26+ cells at day 3 after immunization. (F) WT and GM-CSF−/− mice were injected with GM-CSF−/−/DO11.10 or GM-CSF+/+/DO11.10 CD4+ T cells before immunization with OVA323-339/CFA. Shown is the frequency of apoptotic (Annexin V+ PI−) KJ1-26+ cells at day 3 after immunization. Error bars indicate SD of three mice per group. One representative experiment of two is shown. * , P < 0.05; ** , P < 0.01.
Figure 6.
T cell survival is mediated by DC-secreted factors and IL-6. (A) Expression of GM-CSFR-α, GM-CSFR-β (AIC2B), and IL-2Rβ was determined by quantitative RT-PCR using cDNA of cells indicated. Naive CD62L+ CD4+ T cells (lane 1) and splenic CD11c+ DC (lane 5) were purified by flow cytometry (purity > 98%). Th1 (lane 2), Th2 (lane 3), and Th17 (lane 4) polarized CD4+ T cells. (B and C) DC of GM-CSF−/− or GM-CSF+/+ mice were cultured with purified CD4+ T cells from DO11.10/GM-CSF−/− or DO11.10/GM-CSF+/+ mice, respectively, in the presence of titrating amounts of OVA323-339. (B) IL-6 levels in the culture supernatant at day 3 were determined by ELISA. (C) Proliferation measured by 3H-Thymidine incorporation in the absence and in the presence of 20 ng/ml rIL-6. (D) Splenocytes of DO11.10/GM-CSF+/+ or GM-CSF−/− mice were cultured with 1 μM OVA323-339 in the absence and presence of 20ng/ml rIL-6 for 3 d before staining of cells with KJ1-26+ mAb, Annexin V (AV), and PI and analysis by flow cytometry. Dot plot gated on KJ1-26+ cells shows early apoptotic cells (PI−AV+), late apoptotic (PI+AV+), and dead cells (PI+ AV−). (E) GM-CSF–deficient cultures were supplemented with rIL-1, rIL-2, or rIL-6. Proliferation was measured by 3H-Thymidine incorporation after 3 d of culture. Proliferation index was calculated as described in Materials and methods. (F) CD4+ T cells purified from DO11.10/GM-CSF+/+ or DO11.10/GM-CSF−/− mice were injected i.v. into GM-CSF+/+ or GM-CSF−/− mice, respectively. 2 d later, groups of KO and WT mice were implanted with osmotic minipumps containing hIL-6 or were sham operated. Subsequently, mice were immunized with 200 μg OVA323-339 peptide emulsified in CFA. After 7 d, draining LN and spleen cells were analyzed by flow cytometry. Symbols indicate total number of splenic KJ1-26+ cells of individual mice. Horizontal lines indicate averages of groups. Shown is one representative experiment of three performed. *, < 0.05. Error bars indicate SD.
Figure 7.
GM-CSF−/− mice show reduced frequencies of IL-17+IFN-γ− and IL-17+IFN-γ+ CD4+ T cell populations. (A) CD4+ T cells purified from DO11.10/GM-CSF+/+ or DO11.10/GM-CSF−/− mice were adoptively transferred to GM-CSF+/+ or GM-CSF−/− mice, respectively, 2 d prior to immunization with 200 μg OVA323-339 peptide emulsified in CFA. Mice were boosted at day 7 and draining LN were removed at day 14 to determine KJ1-26+ CD4+ T cells producing IL-17 or IFN-γ by flow cytometry. Top shows dot plots of a representative mouse per group. Bottom shows the frequency of cytokine-producing cells in individual mice. (B and C) GM-CSF−/− and GM-CSF+/+ mice were immunized with myhcα614-629/CFA at days 0 and 7. (B) IL-17 and IFN-γ expression was determined by real-time PCR using cDNA from CD4+ T cells purified from draining LN at day 8. Values show means of groups (n = 3) of mice ±SD. (C) At day 14, CD4+ T cells were isolated from LN and restimulated with myhcα614-629 as described in Materials and methods. IFN-γ– and IL-17–producing cells were determined by ELISPOT analysis. Values indicate individual mice and means of groups.
Figure 8.
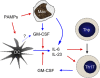
Model for GM-CSF–mediated autoimmunity. PAMPs such as CpG and mycobacterial components in CFA induce DC activation/maturation and production of proinflammatory cytokines, including IL-6, which promote activation of naive CD4 T cells (Thp) and secretion of GM-CSF, IFN-γ, and IL-17. Macrophages (and possibly subpopulations of DC) can produce GM-CSF directly upon encounter of PAMPs. GM-CSF further enhances IL-6 and IL-23 production by DC and macrophages. IL-6 acts at two levels on activated T cells. It enhances survival (both Th1 and Th17) and, together with TGF-β, drives RORγt expression to allow further Th17 polarization. IL-23 can promote Th17 cell maintenance and/or pathogenicity. Blue and red arrows indicate secretion of and stimulation of, respectively.
Similar articles
- Pathogenic IL-23 signaling is required to initiate GM-CSF-driven autoimmune myocarditis in mice.
Wu L, Diny NL, Ong S, Barin JG, Hou X, Rose NR, Talor MV, Čiháková D. Wu L, et al. Eur J Immunol. 2016 Mar;46(3):582-92. doi: 10.1002/eji.201545924. Epub 2016 Jan 12. Eur J Immunol. 2016. PMID: 26660726 Free PMC article. - GM-CSF promotes inflammatory dendritic cell formation but does not contribute to disease progression in experimental autoimmune myocarditis.
Blyszczuk P, Behnke S, Lüscher TF, Eriksson U, Kania G. Blyszczuk P, et al. Biochim Biophys Acta. 2013 Apr;1833(4):934-44. doi: 10.1016/j.bbamcr.2012.10.008. Epub 2012 Oct 24. Biochim Biophys Acta. 2013. PMID: 23103516 - GM-CSF-Producing Th Cells in Rats Sensitive and Resistant to Experimental Autoimmune Encephalomyelitis.
Stojić-Vukanić Z, Pilipović I, Vujnović I, Nacka-Aleksić M, Petrović R, Arsenović-Ranin N, Dimitrijević M, Leposavić G. Stojić-Vukanić Z, et al. PLoS One. 2016 Nov 10;11(11):e0166498. doi: 10.1371/journal.pone.0166498. eCollection 2016. PLoS One. 2016. PMID: 27832210 Free PMC article. - GM-CSF: An immune modulatory cytokine that can suppress autoimmunity.
Bhattacharya P, Thiruppathi M, Elshabrawy HA, Alharshawi K, Kumar P, Prabhakar BS. Bhattacharya P, et al. Cytokine. 2015 Oct;75(2):261-71. doi: 10.1016/j.cyto.2015.05.030. Epub 2015 Jun 22. Cytokine. 2015. PMID: 26113402 Free PMC article. Review. - The link between IL-23 and Th17 cell-mediated immune pathologies.
McGeachy MJ, Cua DJ. McGeachy MJ, et al. Semin Immunol. 2007 Dec;19(6):372-6. doi: 10.1016/j.smim.2007.10.012. Epub 2007 Dec 3. Semin Immunol. 2007. PMID: 18319054 Review.
Cited by
- Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy.
Bhattacharya P, Budnick I, Singh M, Thiruppathi M, Alharshawi K, Elshabrawy H, Holterman MJ, Prabhakar BS. Bhattacharya P, et al. J Interferon Cytokine Res. 2015 Aug;35(8):585-99. doi: 10.1089/jir.2014.0149. Epub 2015 Mar 24. J Interferon Cytokine Res. 2015. PMID: 25803788 Free PMC article. Review. - In vivo Studies on Pharmacokinetics, Toxicity and Immunogenicity of Polyelectrolyte Nanocapsules Functionalized with Two Different Polymers: Poly-L-Glutamic Acid or PEG.
Karabasz A, Szczepanowicz K, Cierniak A, Mezyk-Kopec R, Dyduch G, Szczęch M, Bereta J, Bzowska M. Karabasz A, et al. Int J Nanomedicine. 2019 Dec 5;14:9587-9602. doi: 10.2147/IJN.S230865. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31824153 Free PMC article. - BATF-dependent IL-7RhiGM-CSF+ T cells control intestinal graft-versus-host disease.
Ullrich E, Abendroth B, Rothamer J, Huber C, Büttner-Herold M, Buchele V, Vogler T, Longerich T, Zundler S, Völkl S, Beilhack A, Rose-John S, Wirtz S, Weber GF, Ghimire S, Kreutz M, Holler E, Mackensen A, Neurath MF, Hildner K. Ullrich E, et al. J Clin Invest. 2018 Mar 1;128(3):916-930. doi: 10.1172/JCI89242. Epub 2018 Jan 29. J Clin Invest. 2018. PMID: 29376889 Free PMC article. - Aging diminishes the resistance of AO rats to EAE: putative role of enhanced generation of GM-CSF Expressing CD4+ T cells in aged rats.
Stojić-Vukanić Z, Nacka-Aleksić M, Pilipović I, Vujnović I, Blagojević V, Kosec D, Dimitrijević M, Leposavić G. Stojić-Vukanić Z, et al. Immun Ageing. 2015 Oct 6;12:16. doi: 10.1186/s12979-015-0044-x. eCollection 2015. Immun Ageing. 2015. PMID: 26448779 Free PMC article. - Dendritic cell MST1 inhibits Th17 differentiation.
Li C, Bi Y, Li Y, Yang H, Yu Q, Wang J, Wang Y, Su H, Jia A, Hu Y, Han L, Zhang J, Li S, Tao W, Liu G. Li C, et al. Nat Commun. 2017 Feb 1;8:14275. doi: 10.1038/ncomms14275. Nat Commun. 2017. PMID: 28145433 Free PMC article.
References
- Komiyama, Y., S. Nakae, T. Matsuki, A. Nambu, H. Ishigame, S. Kakuta, K. Sudo, and Y. Iwakura. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 177:566–573. - PubMed
- Nakae, S., A. Nambu, K. Sudo, and Y. Iwakura. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173–6177. - PubMed
- Sonderegger, I., T.A. Rohn, M.O. Kurrer, G. Iezzi, Y. Zou, R.A. Kastelein, M.F. Bachmann, and M. Kopf. 2006. Neutralization of IL-17 by active vaccination inhibits IL-23-dependent autoimmune myocarditis. Eur. J. Immunol. 36:2849–2856. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials