TGF-beta and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3 - PubMed (original) (raw)
TGF-beta and IL-6 signals modulate chromatin binding and promoter occupancy by acetylated FOXP3
Arabinda Samanta et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Expression of FOXP3, a potent gene-specific transcriptional repressor, in regulatory T cells is required to suppress autoreactive and alloreactive effector T cell function. Recent studies have shown that FOXP3 is an acetylated protein in a large nuclear complex and FOXP3 actively represses transcription by recruiting enzymatic corepressors, including histone modification enzymes. The mechanism by which extracellular stimuli regulate the FOXP3 complex ensemble is currently unknown. Although TGF-beta is known to induce murine FOXP3(+) Treg cells, TGF-beta in combination with IL-6 attenuates the induction of FOXP3 functional activities. Here we show that TCR stimuli and TGF-beta signals modulate the disposition of FOXP3 into different subnuclear compartments, leading to enhanced chromatin binding in human CD4(+)CD25(+) regulatory T cells. TGF-beta treatment increases the level of acetylated FOXP3 on chromatin and site-specific recruitment of FOXP3 on the human IL-2 promoter. However, the proinflammatory cytokine IL-6 down-regulates FOXP3 binding to chromatin in the presence of TGF-beta. Moreover, histone deacetylation inhibitor (HDACi) treatment abrogates the down-regulating effects of IL-6 and TGF-beta. These studies indicate that HDACi can enhance regulatory T cell function via promoting FOXP3 binding to chromatin even in a proinflammatory cellular microenvironment. Collectively, our data provide a framework of how different signals affect intranuclear redistribution, posttranslational modifications, and chromatin binding patterns of FOXP3.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
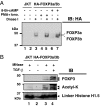
T cell stimulation promotes FOXP3 binding to chromatin. (A) FOXP3 distributes in the cytoplasm (Top), nuclear (Middle), and chromatin (Bottom) fractions after stimulation. Ten million cells transfected with HA-tagged FOXP3a were stimulated with a combination of 50 ng/ml PMA and 1 μM ionomycin or 1 mM 8-bromo-cAMP for the indicated time periods. Cytoplasm, nuclear, and chromatin fractions were prepared from these cells. Equal protein amounts were separated by SDS/PAGE, transferred to nitrocellulose membrane, and immunoblotted with anti-HA-HRP-conjugated antibody, followed by reprobing with anti-β-actin antibody. (B) Stimulation-dependent distribution of FOXP3 in human Treg cells. Ten million human CD4+CD25+ Treg cells were serum starved for 3 h and treated with or without 5 μg of plate-bound anti-CD3 and 2.5 μg of anti-CD28 for the indicated time periods. Equal protein amounts of nuclear and chromatin fractions were separated by SDS/PAGE and immunoblotted with anti-FOXP3 antibody (PCH101, e-bioscience), followed by reprobing with anti-β-actin antibody.
Fig. 2.
TGF-β promotes acetylated FOXP3 binding to chromatin. (A) ChIP was performed by using mIgG, anti-acetyl histone H3 (AcH3), and anti-FOXP3 antibodies with ten million in vitro expanded human CD4+CD25+ Treg cells treated with TGF-β at the indicated time points. The eluted genomic DNA fragment was amplified with hIL-2 promoter-specific primers. (B) Ten million serum-starved human HA-FOXP3a- and -3b-transfected Jurkat T cells were stimulated with or without 1 ng of TGF-β per million cells for indicated time periods. Equal amounts of protein from chromatin fractions were separated by SDS/PAGE, transferred to nitrocellulose, and immunoblotted with anti-acetyl lysine-specific antibody (Ac-K-103, Santa Cruz Biotechnology), followed by reprobing with anti-HA-HRP-conjugated antibody. Acetylated FOXP3a and FOXP3b proteins are indicated.
Fig. 3.
Nuclease digestions released acetylated FOXP3 from chromatin. (A) Ten million serum-starved transfected or control cells were stimulated with a combination of 50 ng/ml PMA and 1 μM ionomycin or 1 mM 8-bromo-cAMP alone for the indicated time. Isolated chromatin fractions were digested with 20 units of DNase I. The released chromatin-bound proteins in solution were separated by SDS/PAGE, transferred, and immunobloted with anti-HA-HRP conjugated antibody. (B) MNase-mediated release of acetylated FOXP3 from chromatin. Ten million HA-FOXP3a- and -3b-transfected or untransfected Jurkat T cells were stimulated as indicated. The chromatin fractions were digested with 10 units of MNase (Roche), the released chromatin digested material was tested for FOXP3 by immunoblotting with anti-HA-HRP antibody, and its acetylation was detected by immunoblotting with anti-acetyl lysine-specific mAb-Ac-K-103 (Upstate Biotechnology).
Fig. 4.
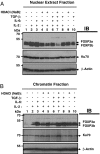
IL-6 plus TGF-β down-regulates FOXP3 binding to chromatin that could be reversed by HDACi sodium butyrate. Ten million FOXP3+ SZ-4 T cells were stimulated in the presence or absence of HDACi (10 mM sodium butyrate) along with 20 ng/ml IL-6, 5 ng/ml TGF-β, and 50 U/ml IL-2 as indicated for 4 h in 10% FBS containing RPMI-1640 medium supplied with 10 U/ml IL-2. Cells were washed with cold PBS containing all inhibitors and fractionated into cytoplasmic, nuclear, and chromatin fractions. Equal amounts of proteins were separated by 8% SDS/PAGE, then transferred to nitrocellulose membrane. Both the nuclear fraction (A) and chromatin fraction (B) were immunoblotted successively with antibodies against FOXP3 (221D), Ku-70 (Santa Cruz Biotechnology, sc-17789), and β-actin (Sigma).
Similar articles
- Synergy between IL-6 and TGF-β signaling promotes FOXP3 degradation.
Gao Z, Gao Y, Li Z, Chen Z, Lu D, Tsun A, Li B. Gao Z, et al. Int J Clin Exp Pathol. 2012;5(7):626-33. Epub 2012 Sep 5. Int J Clin Exp Pathol. 2012. PMID: 22977658 Free PMC article. - STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor.
Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. Takaki H, et al. J Biol Chem. 2008 May 30;283(22):14955-62. doi: 10.1074/jbc.M801123200. Epub 2008 Apr 9. J Biol Chem. 2008. PMID: 18400747 Free PMC article. - JunB and c-Rel cooperatively enhance Foxp3 expression during induced regulatory T cell differentiation.
Son JS, Sahoo A, Chae CS, Hwang JS, Park ZY, Im SH. Son JS, et al. Biochem Biophys Res Commun. 2011 Apr 1;407(1):141-7. doi: 10.1016/j.bbrc.2011.02.126. Epub 2011 Mar 1. Biochem Biophys Res Commun. 2011. PMID: 21371435 - The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road.
Zhang L, Zhao Y. Zhang L, et al. J Cell Physiol. 2007 Jun;211(3):590-7. doi: 10.1002/jcp.21001. J Cell Physiol. 2007. PMID: 17311282 Review. - Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other.
Horwitz DA, Zheng SG, Gray JD. Horwitz DA, et al. Trends Immunol. 2008 Sep;29(9):429-35. doi: 10.1016/j.it.2008.06.005. Epub 2008 Aug 3. Trends Immunol. 2008. PMID: 18676178 Review.
Cited by
- Treating arthritis by immunomodulation: is there a role for regulatory T cells?
Wehrens EJ, van Wijk F, Roord ST, Albani S, Prakken BJ. Wehrens EJ, et al. Rheumatology (Oxford). 2010 Sep;49(9):1632-44. doi: 10.1093/rheumatology/keq130. Epub 2010 May 12. Rheumatology (Oxford). 2010. PMID: 20463189 Free PMC article. Review. - BATF is Required for Treg Homeostasis and Stability to Prevent Autoimmune Pathology.
Khatun A, Wu X, Qi F, Gai K, Kharel A, Kudek MR, Fraser L, Ceicko A, Kasmani MY, Majnik A, Burns R, Chen YG, Salzman N, Taparowsky EJ, Fang D, Williams CB, Cui W. Khatun A, et al. Adv Sci (Weinh). 2023 Oct;10(28):e2206692. doi: 10.1002/advs.202206692. Epub 2023 Aug 16. Adv Sci (Weinh). 2023. PMID: 37587835 Free PMC article. - TRAF6 directs FOXP3 localization and facilitates regulatory T-cell function through K63-linked ubiquitination.
Ni X, Kou W, Gu J, Wei P, Wu X, Peng H, Tao J, Yan W, Yang X, Lebid A, Park BV, Chen Z, Tian Y, Fu J, Newman S, Wang X, Shen H, Li B, Blazar BR, Wang X, Barbi J, Pan F, Lu L. Ni X, et al. EMBO J. 2019 May 2;38(9):e99766. doi: 10.15252/embj.201899766. Epub 2019 Mar 18. EMBO J. 2019. PMID: 30886050 Free PMC article. - An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes.
Darce J, Rudra D, Li L, Nishio J, Cipolletta D, Rudensky AY, Mathis D, Benoist C. Darce J, et al. Immunity. 2012 May 25;36(5):731-41. doi: 10.1016/j.immuni.2012.04.007. Epub 2012 May 10. Immunity. 2012. PMID: 22579475 Free PMC article. - Lymphotoxin-beta receptor blockade induces inflammation and fibrosis in tolerized cardiac allografts.
Nakayama Y, Bromberg JS. Nakayama Y, et al. Am J Transplant. 2012 Sep;12(9):2322-34. doi: 10.1111/j.1600-6143.2012.04090.x. Epub 2012 May 17. Am J Transplant. 2012. PMID: 22594431 Free PMC article.
References
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
- Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: Regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. - PubMed
- Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials