Decyanation of vitamin B12 by a trafficking chaperone - PubMed (original) (raw)
Decyanation of vitamin B12 by a trafficking chaperone
Jihoe Kim et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The mystery of how the cyanide group in vitamin B(12) or cyanocobalamin, discovered 60 years ago, is removed, has been solved by the demonstration that the trafficking chaperone, MMACHC, catalyzes a reductive decyanation reaction. Electrons transferred from NADPH via cytosolic flavoprotein oxidoreductases are used to cleave the cobalt-carbon bond with reductive elimination of the cyanide ligand. The product, cob(II)alamin, is a known substrate for assimilation into the active cofactor forms, methylcobalamin and 5'-deoxyadenosylcobalamin, and is bound in the "base-off" state that is needed by the two B(12)-dependent target enzymes, methionine synthase and methylmalonyl-CoA mutase. Defects in MMACHC represent the most common cause of inborn errors of B(12) metabolism, and our results explain the observation that fibroblasts from these patients are poorly responsive to vitamin B(12) but show some metabolic correction with aquocobalamin, a cofactor form lacking the cyanide ligand, which is mirrored by patients showing poorer clinical responsiveness to cyano- versus aquocobalamin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
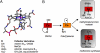
Structures of B12 derivatives. (A) Structure of free base-on cobalamin showing that five of the six ligands are provided by the corrin ring. The lower axial ligand is the endogenous base, dimethylbenzimidazole. The upper axial ligand is variable, as indicated. MeCbl, AdoCbl, and H2OCbl refer to methylcobalamin, deoxyadenosylcobalamin, and aquocobalamin respectively. (B) Conversion of vitamin B12 to the active cofactor forms of B12 requires a decyanation step. The product, cob(II)alamin, can be used for the synthesis of MeCbl or AdoCbl, which are bound in the base-off/His-on state in methionine synthase and methylmalonyl-CoA mutase, respectively. The differences in the UV-visible absorption spectra for these cobalamins lead to orange or red compounds as denoted.
Fig. 2.
Binding of CNCbl to MMACHC. (A) UV-visible absorption spectra of 10 μM free CNCbl (dashed line) or bound to 20 μM MMACHC (solid line) in 100 mM Hepes (pH 7.0), 150 mM KCl, and 5% glycerol. (Inset) Comparison between base-on (dashed line) and base-off (solid line) CNCbl in water and 6% HClO4, respectively. (B) Binding of CNCbl to MMACHC was monitored by isothermal titration calorimetry of 25 μM MMACHC with CNCbl (1–500 μM) as described in Materials and Methods. Analysis of the titration data reveals stoichiometric binding and yields a _K_d of 15.5 ± 1.1 μM.
Fig. 3.
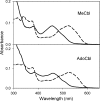
Binding of methyl- or 5′-deoxyadenosylcobalamin to MMACHC. UV-visible absorption spectra of 10 μM free methyl- or 5′-deoxyadenosylcobalamin (dashed line) or bound to 20 μM MMACHC (solid line) in 100 mM Hepes (pH 7.0), 150 mM KCl, and 5% glycerol are shown.
Fig. 4.
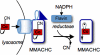
Decyanation of CNCbl by MMACHC. (A) Spectrum of MMACHC reconstituted with CNCbl (blue) and its conversion to cob(II)alamin (red) in the presence of methionine synthase reductase and NADPH. (Inset) Rate of cob(II)alamin formation from 20 μM MMACHC/CNCbl in the presence of 4 μM methionine synthase reductase (●), 4 μM NR1 (▴), or in the absence of MMACHC (open symbols). (B) EPR spectra of cob(II)alamin (100 μM) generated in solution or formed by decyanation of 100 μM MMACHC/CNCbl as described in Materials and Methods.
Fig. 5.
Model for the decyanase activity of MMACHC. The dark blue domain in MMACHC represents the C-terminal TonB-like domain, which is proposed to open the “hatch” on the lysosomal transporter, in analogy with the role of TonB in bacterial two-component transport systems (6, 12).
Similar articles
- A human vitamin B12 trafficking protein uses glutathione transferase activity for processing alkylcobalamins.
Kim J, Hannibal L, Gherasim C, Jacobsen DW, Banerjee R. Kim J, et al. J Biol Chem. 2009 Nov 27;284(48):33418-24. doi: 10.1074/jbc.M109.057877. Epub 2009 Oct 2. J Biol Chem. 2009. PMID: 19801555 Free PMC article. - Redox-Linked Coordination Chemistry Directs Vitamin B12 Trafficking.
Banerjee R, Gouda H, Pillay S. Banerjee R, et al. Acc Chem Res. 2021 Apr 20;54(8):2003-2013. doi: 10.1021/acs.accounts.1c00083. Epub 2021 Apr 2. Acc Chem Res. 2021. PMID: 33797888 Free PMC article. - The human B12 trafficking protein CblC processes nitrocobalamin.
Mascarenhas R, Li Z, Gherasim C, Ruetz M, Banerjee R. Mascarenhas R, et al. J Biol Chem. 2020 Jul 10;295(28):9630-9640. doi: 10.1074/jbc.RA120.014094. Epub 2020 May 26. J Biol Chem. 2020. PMID: 32457044 Free PMC article. - Intracellular processing of vitamin B12 by MMACHC (CblC).
Hannibal L, Jacobsen DW. Hannibal L, et al. Vitam Horm. 2022;119:275-298. doi: 10.1016/bs.vh.2022.02.001. Epub 2022 Mar 15. Vitam Horm. 2022. PMID: 35337623 Review. - Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency.
Obeid R, Fedosov SN, Nexo E. Obeid R, et al. Mol Nutr Food Res. 2015 Jul;59(7):1364-72. doi: 10.1002/mnfr.201500019. Epub 2015 May 12. Mol Nutr Food Res. 2015. PMID: 25820384 Free PMC article. Review.
Cited by
- An Interprotein Co-S Coordination Complex in the B12-Trafficking Pathway.
Li Z, Mascarenhas R, Twahir UT, Kallon A, Deb A, Yaw M, Penner-Hahn J, Koutmos M, Warncke K, Banerjee R. Li Z, et al. J Am Chem Soc. 2020 Sep 23;142(38):16334-16345. doi: 10.1021/jacs.0c06590. Epub 2020 Sep 14. J Am Chem Soc. 2020. PMID: 32871076 Free PMC article. - Vitamin B12 transport from food to the body's cells--a sophisticated, multistep pathway.
Nielsen MJ, Rasmussen MR, Andersen CB, Nexø E, Moestrup SK. Nielsen MJ, et al. Nat Rev Gastroenterol Hepatol. 2012 May 1;9(6):345-54. doi: 10.1038/nrgastro.2012.76. Nat Rev Gastroenterol Hepatol. 2012. PMID: 22547309 Review. - Structural basis of multifunctionality in a vitamin B12-processing enzyme.
Koutmos M, Gherasim C, Smith JL, Banerjee R. Koutmos M, et al. J Biol Chem. 2011 Aug 26;286(34):29780-7. doi: 10.1074/jbc.M111.261370. Epub 2011 Jun 22. J Biol Chem. 2011. PMID: 21697092 Free PMC article. - Vitamin B12 Metabolism: A Network of Multi-Protein Mediated Processes.
Mucha P, Kus F, Cysewski D, Smolenski RT, Tomczyk M. Mucha P, et al. Int J Mol Sci. 2024 Jul 23;25(15):8021. doi: 10.3390/ijms25158021. Int J Mol Sci. 2024. PMID: 39125597 Free PMC article. Review. - Guided cobalamin biosynthesis supports Dehalococcoides mccartyi reductive dechlorination activity.
Yan J, Im J, Yang Y, Löffler FE. Yan J, et al. Philos Trans R Soc Lond B Biol Sci. 2013 Mar 11;368(1616):20120320. doi: 10.1098/rstb.2012.0320. Print 2013 Apr 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23479750 Free PMC article.
References
- Rickes EL, Brink NG, Konivszy FR, Wood TR, Folkers K. Crystalline vitamin B12. Science. 1948;107:396–397. - PubMed
- Smith EL. Purification of anti-pernicious anemia factors from liver. Nature. 1948;161:638. - PubMed
- Hodgkin DC, et al. Structure of vitamin B12. Nature. 1956;178:64–66. - PubMed
- Rosenblatt DS, Fenton WA. In: The Metabolic and Molecular Bases of Inherited Diseases. Scriver CR, Sly WS, editors. New York: McGraw–Hill; 2001. pp. 3897–3933.
- Hsu JM, Kawin B, Minor P, Mitchell JA. Vitamin B12 concentrations in human tissue. Nature. 1966;210:1264–1265.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases