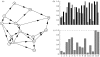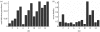Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities - PubMed (original) (raw)
Connectivity sustains disease transmission in environments with low potential for endemicity: modelling schistosomiasis with hydrologic and social connectivities
David Gurarie et al. J R Soc Interface. 2009.
Abstract
Social interaction and physical interconnections between populations can influence the spread of parasites. The role that these pathways play in sustaining the transmission of parasitic diseases is unclear, although increasingly realistic metapopulation models are being used to study how diseases persist in connected environments. We use a mathematical model of schistosomiasis transmission for a distributed set of heterogeneous villages to show that the transport of parasites via social (host movement) and environmental (parasite larvae movement) pathways has consequences for parasite control, spread and persistence. We find that transmission can be sustained regionally throughout a group of connected villages even when individual village conditions appear not to support endemicity. Optimum transmission is determined by an interplay between different transport pathways, and not necessarily by those that are the most dispersive (e.g. disperse social contacts may not be optimal for transmission). We show that the traditional targeting of villages with high infection, without regard to village interconnections, may not lead to optimum control. These findings have major implications for effective disease control, which needs to go beyond considering local variations in disease intensity, to also consider the degree to which populations are interconnected.
Figures
Figure 1
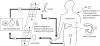
Schistosomiasis transmission. (1) Humans can become infected by the free-swimming cercarial form of the parasite from skin contact with contaminated surface water. (2) Once infected, adult schistosome worms will develop in the blood vessels of the liver or bladder depending on the species of schistosome. Male and female worms will mate and produce eggs, which are released in either the faeces or urine. (3) Inadequate sanitation results in parasitic eggs being deposited into an aquatic environment (e.g. ponds, irrigation ditches), where they hatch into a free-swimming miracidial form of the parasite. (4) Miracidia infect the snail intermediate host, which lives in these aquatic environments. (5) After a prepatency period, infected snails will begin to release cercariae, which infect humans to complete the parasite's life cycle. (6) Both cercarial and miracidial forms of the parasite may be transported between communities via hydrologic connections such as irrigation ditches. (7) Hosts may also transport the parasite between communities via travel, migration and mobile labour (infection and/or contamination within and outside of the host's own village).
Figure 2
(a) Model environment of 15 hydrologically connected villages. Relative human and snail populations at each site are shown in (b) (black bars, human; grey bars, snail), along with (c) the resulting local basic reproduction number (local BRN)—all but site #14 are below sustainable level 1 (dashed line). The sites are numbered in the ‘down-stream’ (partial) order.
Figure 3
Distribution of (a) equilibrium snail infection prevalences (as a proportion) and (b) worm burdens by village. Sustained transmission occurs in all 15 villages at moderate hydrologic transport (_β_=5), wide social contact dispersion (_α_=5) and high throughput rate _r_=1, despite only site #14 in figure 1 with local BRN=1.
Figure 4
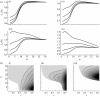
The effect of social and hydrological connectivities on persistence of endemic infection. (a) Eigenvalue _λ_1(R) over the range of social contact dispersion 1<_α_<30 for four hydrologic transport _β_=4; 5; 6; 7 (top-to-bottom curves). (i–iv) follow changing hydrologic throughput from low throughput _r_=0.4 (i) to high throughput _r_=1 (iv). (_b_(i)–(iii)) shows the isocontours of the eigenvalue _λ_1(_R_) in the (_r_,_α_) parameter space for three values of _β_=2 (i) to _β_=6 (iii) (shaded isocontours represent _λ_1(_R_)>1 with darker shades of grey indicating higher values _λ_1(R)). In (a,b), we observe how different choices of hydrologic parameters r, β create distinct ranges of social contact dispersion α for persistent infection (_λ_1(R)>1).
Figure 5
(a(i)–(iii)) Equilibrium snail infection prevalences and (b(i)–(iii)) rescaled worm burdens over 15 village sites for varying levels of social contact dispersion 1<α<30, at high throughput r_=0.9. (a(i)–_b(i)) The snail infection prevalences and worm burdens for β_=2 (fast hydrologic transport), (a(ii)–_b(ii)) β_=3 (medium hydrologic transport) and (a(iii)–_b(iii)) _β_=5 (slow hydrologic transport).
Figure 6
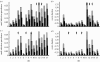
Local molluscicide control. (a,c) Snail infection prevalence and (b,d) worm burden. (a,b) Focal control at sites #11, 12 and 13: at 100% snail density (light grey), 50% reduction (dark grey), 0% complete removal (black). (c,d) Similar control at sites #4, 7 and 9 with 100% snail density (light grey), and reductions of 30% (dark grey), 60% (black) and 90% (invisible—elimination).
References
- Anderson R.M., May R.M. Oxford University Press; New York, NY: 1991. Infectious diseases of humans: dynamics and control.
- Appleton C.C., Ngxongo S.M., Braack L.E., le Sueur D. Schistosoma mansoni in migrants entering South Africa from Mocambique—a threat to public health in north-eastern KwaZulu-Natal? S. Afr. Med. J. 1996;86:350–353. - PubMed
- Barbera J., Macintyre A., Gostin L., Inglesby T., O'Toole T., DeAtley C., Tonat K., Layton M. Large-scale quarantine following biological terrorism in the United States—scientific examination, logistic and legal limits, and possible consequences. J. Am. Med. Assoc. 2001;286:2711–2717. doi: 10.1001/jama.286.21.2711. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 TW001543/TW/FIC NIH HHS/United States
- R01 AI050612/AI/NIAID NIH HHS/United States
- R01 AI068854/AI/NIAID NIH HHS/United States
- R01 AI50612/AI/NIAID NIH HHS/United States
- R01 TW001543-05S1/TW/FIC NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical