Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain - PubMed (original) (raw)
Functional connectivity MR imaging reveals cortical functional connectivity in the developing brain
W Lin et al. AJNR Am J Neuroradiol. 2008 Nov.
Abstract
Background and purpose: Unlike conventional functional MR imaging where external sensory/cognitive paradigms are needed to specifically activate different regions of the brain, resting functional connectivity MR imaging acquires images in the absence of cognitive demands (a resting condition) and detects brain regions, which are highly temporally correlated. Therefore, resting functional MR imaging is highly suited for the study of brain functional development in pediatric subjects. This study aimed to determine the temporal and spatial patterns of rfc in healthy pediatric subjects between 2 weeks and 2 years of age.
Materials and methods: Rfc studies were performed on 85 children: 38 neonates (2-4 weeks of age), 26 one-year-olds, and 21 two-year-olds. All subjects were imaged while asleep; no sedation was used. Six regions of interest were chosen, including the primary motor, sensory, and visual cortices in each hemisphere. Mean signal intensity of each region of interest was used to perform correlation analysis pixel by pixel throughout the entire brain, identifying regions with high temporal correlation.
Results: Functional connectivity was observed in all subjects in the sensorimotor and visual areas. The percent brain volume exhibiting rfc and the strength of rfc continued to increase from 2 weeks to 2 years. The growth trajectories of the percent brain volume of rfc appeared to differ between the sensorimotor and visual areas, whereas the z-score was similar. The percent brain volume of rfc in the sensorimotor area was significantly larger than that in the visual area for subjects 2 weeks of age (P = .008) and 1-year-olds (P = .017) but not for the 2-year-olds.
Conclusions: These findings suggest that rfc in the sensorimotor precedes that in the visual area from 2 weeks to 1 year but becomes comparable at 2 years. In contrast, the comparable z-score values between the sensorimotor and visual areas for all age groups suggest a disassociation between percent brain volume and the strength of cortical rfc.
Figures
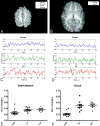
Fig 1.
A and B, The anatomic locations for defining the sensorimotor and visual areas are shown, respectively. The crosses, filled circles, squares, asterisks, left triangles, and right triangles represent the right motor, right sensory, left motor, left sensory, left visual, and right visual cortices, respectively. C and D, Representative processed MR signals at the sensorimotor and visual areas by using the approaches outlined in the “Materials and Methods” section for a neonate (upper row) and 1- (middle row) and 2-year-old (bottom row) children are shown, respectively. E and F, The percent signal intensity difference between the maximum and minimum signals for the sensorimotor and visual areas are shown for each age group. The error bars represent the SDs. max indicates maximum; s, second; wk, week; yr, year.
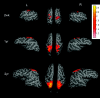
Fig 2.
The averaged group results demonstrate the brain regions exhibiting rfc when the right and left sensorimotor regions of interest are used to obtain the reference functions for correlation analysis and are superimposed on the brain surface for neonates and the 1- and 2-year-old groups. The color bar represents the _z_-score values. L indicates left hemisphere; R, right hemisphere; wk, week; yr, year.
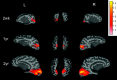
Fig 3.
The averaged group results demonstrate the brain regions exhibiting rfc when the right and left visual regions of interest are used to obtain the reference functions for correlation analysis and are superimposed on the brain surface for neonates and the 1- and 2-year-old groups. The color bar represents the _z_-score values. L indicates left hemisphere; R, right hemisphere; wk, week; yr, year.
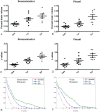
Fig 4.
Quantitative comparisons of the percent brain volume exhibiting rfc (A and B) and the _z_-score values (C and D) and histograms (E and F) for the 3 age groups at both the sensorimotor and visual areas are shown. A_–_D, The error bars represent the SDs. E and F, The y-axes represent the normalized populations. yr indicates year; wk, week.
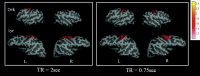
Fig 5.
A comparison of 2 different TRs for obtaining rfc at the sensorimotor area is shown. The left and right sensorimotor regions of interest are used to obtain the reference functions for correlation analysis for both TRs. The color bar represents the _z_-score values. L indicates left hemisphere; R, right hemisphere; wk, week; yr, year.
Similar articles
- Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest.
van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE. van de Ven VG, et al. Hum Brain Mapp. 2004 Jul;22(3):165-78. doi: 10.1002/hbm.20022. Hum Brain Mapp. 2004. PMID: 15195284 Free PMC article. - Disrupted functional connectivity between sub-regions in the sensorimotor areas and cortex in migraine without aura.
Qin Z, Su J, He XW, Ban S, Zhu Q, Cui Y, Zhang J, Hu Y, Liu YS, Zhao R, Qiao Y, Li J, Liu JR, Du X. Qin Z, et al. J Headache Pain. 2020 May 6;21(1):47. doi: 10.1186/s10194-020-01118-1. J Headache Pain. 2020. PMID: 32375638 Free PMC article. - Functional connectivity of the sensorimotor area in naturally sleeping infants.
Liu WC, Flax JF, Guise KG, Sukul V, Benasich AA. Liu WC, et al. Brain Res. 2008 Aug 5;1223:42-9. doi: 10.1016/j.brainres.2008.05.054. Epub 2008 May 28. Brain Res. 2008. PMID: 18599026 - Longitudinal Study of the Emerging Functional Connectivity Asymmetry of Primary Language Regions during Infancy.
Emerson RW, Gao W, Lin W. Emerson RW, et al. J Neurosci. 2016 Oct 19;36(42):10883-10892. doi: 10.1523/JNEUROSCI.3980-15.2016. J Neurosci. 2016. PMID: 27798142 Free PMC article. - Neuromagnetic integrated methods tracking human brain mechanisms of sensorimotor areas 'plastic' reorganisation.
Rossini PM, Pauri F. Rossini PM, et al. Brain Res Brain Res Rev. 2000 Sep;33(2-3):131-54. doi: 10.1016/s0169-328x(00)00090-5. Brain Res Brain Res Rev. 2000. PMID: 11011062 Review.
Cited by
- Mapping region-specific longitudinal cortical surface expansion from birth to 2 years of age.
Li G, Nie J, Wang L, Shi F, Lin W, Gilmore JH, Shen D. Li G, et al. Cereb Cortex. 2013 Nov;23(11):2724-33. doi: 10.1093/cercor/bhs265. Epub 2012 Aug 23. Cereb Cortex. 2013. PMID: 22923087 Free PMC article. - Longitudinal Infant Functional Connectivity Prediction via Conditional Intensive Triplet Network.
Yu X, Hu D, Zhang L, Huang Y, Wu Z, Liu T, Wang L, Lin W, Zhu D, Li G. Yu X, et al. Med Image Comput Comput Assist Interv. 2022 Sep;13438:255-264. doi: 10.1007/978-3-031-16452-1_25. Epub 2022 Sep 16. Med Image Comput Comput Assist Interv. 2022. PMID: 36563062 Free PMC article. - The Impact of Caregiving on the Association Between Infant Emotional Behavior and Resting State Neural Network Functional Topology.
Hanford LC, Schmithorst VJ, Panigrahy A, Lee V, Ridley J, Bonar L, Versace A, Hipwell AE, Phillips ML. Hanford LC, et al. Front Psychol. 2018 Oct 15;9:1968. doi: 10.3389/fpsyg.2018.01968. eCollection 2018. Front Psychol. 2018. PMID: 30374323 Free PMC article. - Resting-state functional MRI studies on infant brains: A decade of gap-filling efforts.
Zhang H, Shen D, Lin W. Zhang H, et al. Neuroimage. 2019 Jan 15;185:664-684. doi: 10.1016/j.neuroimage.2018.07.004. Epub 2018 Jul 7. Neuroimage. 2019. PMID: 29990581 Free PMC article. Review. - Clinical and Preclinical Evidence for Adverse Neurodevelopment after Postnatal Zika Virus Infection.
Raper J, Chahroudi A. Raper J, et al. Trop Med Infect Dis. 2021 Jan 12;6(1):10. doi: 10.3390/tropicalmed6010010. Trop Med Infect Dis. 2021. PMID: 33445671 Free PMC article. Review.
References
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 1997;10:165–70 - PubMed
- Biswal B, Hudetz AG, Yetkin FZ, et al. Hypercapnia reversibly suppresses low-frequency fluctuations in the human motor cortex during rest using echo-planar MRI. J Cereb Blood Flow Metab 1997;17:301–08 - PubMed
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 1998;7:119–32 - PubMed
- Lowe MJ, Dzemidzic M, Lurito JT, et al. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage 2000;12:582–87 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical