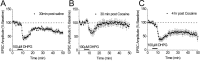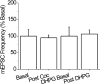In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression - PubMed (original) (raw)
In vivo metabotropic glutamate receptor 5 (mGluR5) antagonism prevents cocaine-induced disruption of postsynaptically maintained mGluR5-dependent long-term depression
Brad A Grueter et al. J Neurosci. 2008.
Abstract
Metabotropic glutamate receptor 5 (mGluR5) plays a critical role in psychostimulant-induced behavior, yet it is unclear whether mGluR5 is activated by psychostimulant administration, or whether its role is constitutive. We previously reported that activation of mGluR5 with the group I mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) can induce a long-term depression (DHPG-LTD) of glutamatergic transmission in the bed nucleus of the stria terminalis (BNST), and that ex vivo induction of this LTD is disrupted by repeated in vivo administration of cocaine. Here we demonstrate that DHPG-LTD is not maintained by alterations in glutamate release, and that postsynaptic endocytosis is necessary. Furthermore, we find that a single administration of cocaine produces a transient disruption of DHPG-LTD, and the duration of this disruption was increased by repeated days of cocaine administration. The disruption produced by cocaine was not permanent, because DHPG-LTD could be induced 10 d after cocaine administration. To test the role of mGluR5 in vivo in the cocaine-induced disruption of DHPG-LTD, we injected mice with the mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine before cocaine. mGluR5 antagonism during in vivo cocaine administration rescued subsequent ex vivo induction of DHPG-LTD. The effects of in vivo cocaine could be mimicked by application of cocaine to BNST-containing slices, suggesting that the actions of cocaine are local. Thus, using a novel strategy of in vivo antagonist-induced rescue of ex vivo agonist effects for the same receptor, we provide evidence suggesting that mGluR5 activation is actively recruited by in vivo cocaine.
Figures
Figure 1.
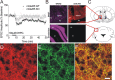
mGluR5-LTD in the BNST. A, Group I mGluR LTD in the BNST is dependent on mGluR5 function. Average time courses of normalized EPSC amplitudes evoked by local stimulation in the BNST in acute slice preparations are shown. DHPG (100 μ
m
) was applied for 5 min as indicated. ○, mGluR5 knock-out; ■, wild-type littermates. B, Mouse brain coronal slices containing the dorsal BNST (top) or a portion of the cerebellum (bottom) stained with DRAQ nuclear stain (left) or anti-mGluR5 (right) show a specific mGluR5 signal in the BNST. AC, Anterior commissure; Str, striatum; AL, ansiform lobule; F1, fissure 1. C, Schematic depicting the region contained in the micrographs (red squares). Dorsal BNST in blue (top), cerebellum (bottom). D–F, Mouse brain coronal slices were stained with anti-mGluR5 (D; red) or the dendritic marker anti-MAP2 (E; green) (F; merge). Scale bar, 20 μm.
Figure 2.
A, B, Normalized time course of effects of DHPG on EPSC amplitude in external ACSF containing 1 m
m
Ca2+/2.8 m
m
Mg2+ (A) or 1 m
m
Ca2+/1 m
m
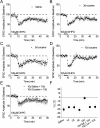
Mg2+ (B). C, Summary graph of effects of varying extracellular cations on average basal PPR (EPSC2/EPSC1). *p < 0.05. D, Varying extracellular cations did not affect PPR normalized to baseline 30 min after DHPG washout. E, Representative experiment depicting time course of γ-DGG-induced depression of EPSCs before and after DHPG application. F, Percentage of inhibition by γ-DGG is unchanged before and after DHPG application. Error bars indicate SEM.
Figure 3.
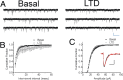
DHPG decreased the frequency but not the amplitude of BNST mEPSCs. A, Sample current traces of mEPSCs in a BNST neuron before DHPG application (basal) and at 30 min after (late; LTD) DHPG. Calibration: 20 pA, 512 ms. B, Cumulative probability plot of DHPG induced effects on mEPSC inter-event interval within the basal (3–5 min, open squares) and late (LTD; min 35–37, closed circles) time periods (n = 11; p < 0.05, Kolmogorov–Smirnov test). C, Cumulative probability of effects of DHPG on mEPSC amplitude within the same current recordings as shown in B. Inset, Representative traces before DHPG application and during DHPG-mediated LTD. Inset calibration, 5 pA. Error bars indicate SEM.
Figure 4.
Maintenance of mGluR5 LTD is dependent on postsynaptic endocytosis machinery. A, Average time course of effects of 100 μ
m
DHPG on normalized EPSC amplitudes from BNST neurons perfused with 2 m
m
dynamin-inhibitory peptide. B, Time course of effects of 100 μ
m
DHPG on EPSCs from BNST neurons postsynaptically perfused with either jasplakinolide (2 μ
m)
(○) or vehicle (DMSO) (■). n = 7 (1 cell was excluded from this analysis because DHPG produced a depression of the EPSC twice the normal observed amplitude in naive neurons). Error bars indicate SEM.
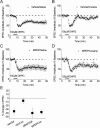
Figure 5.
Repeated exposure to cocaine attenuates group I mGluR LTD in the BNST. A–E, Average time courses of normalized EPSC amplitudes evoked by local stimulation in the BNST in slices taken from saline and cocaine (20 mg/kg)-treated mice. DHPG (100 μ
m
) was applied for 5 min as indicated. Specific mouse treatments were as follows: A, mice killed and slices prepared 24 h after 10 d of intraperitoneal saline injections; B–D, mice killed and slices prepared 24 h after 3, 5, and 10 d of intraperitoneal cocaine injections, respectively; E, mice killed and slices prepared 10 d after 10 d of intraperitoneal cocaine or saline injections. F, Summary graph in which values represent percentage change in EPSC ± SEM, 30 min after DHPG washout. *p < 0.05 (Bonferroni and Tukey test after one-way ANOVA). Error bars indicate SEM.
Figure 6.
A single cocaine exposure transiently attenuates group I mGluR LTD in the BNST. A–C, Average time courses of normalized EPSC amplitudes evoked by local stimulation in the BNST in slices taken from saline and cocaine (20 mg/kg)-treated mice 30 min (A, B) or 4 h (C) after injection. DHPG (100 μ
m
) was applied for 5 min as indicated.
Figure 7.
Cocaine has local effects on mGluR5 signaling in BNST. Summary graph representing percentage change in mEPSC frequency after bath application of 30 μ
m
cocaine (Coc) and 30 min after subsequent DHPG (100 μ
m
) application.
Figure 8.
Repeated cocaine-induced changes in group I mGluR LTD are dependent on mGluR5 function in vivo. A–D, Average time courses of normalized EPSC amplitudes evoked by local stimulation in the BNST in acute slice preparations. DHPG (100 μ
m
) was applied for 5 min as indicated. In each case, mice underwent 10 d of intraperitoneal injections and were killed 24 h after the last injection. Specific daily mouse treatments were as follows: A, intraperitoneal vehicle followed 30 min later by intraperitoneal saline; B, intraperitoneal vehicle followed 30 min later by intraperitoneal cocaine (20 mg/kg); C, intraperitoneal MPEP (10 mg/kg) followed 30 min later by intraperitoneal saline; D, intraperitoneal MPEP (10 mg/kg) followed 30 min later by intraperitoneal cocaine (20 mg/kg). E, Summary graph in which values represent percentage change in EPSC ± SEM, 30 min after DHPG washout. *p < 0.05 (Bonferroni and Tukey test after one-way ANOVA). Error bars indicate SEM.
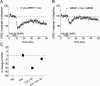
Figure 9.
A single cocaine exposure alters mGluR5 LTD in BNST slices in an mGluR5-dependent manner on an acute (30 min after cocaine) time scale. A–B, Average time courses of normalized EPSC amplitudes evoked by local stimulation in the BNST in acute slice preparations. DHPG (100 μ
m
) was applied for 5 min as indicated. A, Intraperitoneal MPEP (10 mg/kg) followed 30 min later by intraperitoneal cocaine (Coc) (20 mg/kg). In B, mice were conditioned as in A, and MPEP (10 μ
m
) was then applied to the slice 10 min before DHPG application. C, Summary graph in which values represent percentage change in EPSC ± SEM, 30 min after DHPG washout. *p < 0.05 (Bonferroni and Tukey test after one-way ANOVA). Error bars indicate SEM. Values taken from time courses in A and B and Figure 6, A and B.
Similar articles
- Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors.
O'Riordan KJ, Huang IC, Pizzi M, Spano P, Boroni F, Egli R, Desai P, Fitch O, Malone L, Ahn HJ, Liou HC, Sweatt JD, Levenson JM. O'Riordan KJ, et al. J Neurosci. 2006 May 3;26(18):4870-9. doi: 10.1523/JNEUROSCI.4527-05.2006. J Neurosci. 2006. PMID: 16672661 Free PMC article. - Extracellular-signal regulated kinase 1-dependent metabotropic glutamate receptor 5-induced long-term depression in the bed nucleus of the stria terminalis is disrupted by cocaine administration.
Grueter BA, Gosnell HB, Olsen CM, Schramm-Sapyta NL, Nekrasova T, Landreth GE, Winder DG. Grueter BA, et al. J Neurosci. 2006 Mar 22;26(12):3210-9. doi: 10.1523/JNEUROSCI.0170-06.2006. J Neurosci. 2006. PMID: 16554472 Free PMC article. - Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression.
Volk LJ, Daly CA, Huber KM. Volk LJ, et al. J Neurophysiol. 2006 Apr;95(4):2427-38. doi: 10.1152/jn.00383.2005. Epub 2006 Jan 18. J Neurophysiol. 2006. PMID: 16421200 - Cocaine Withdrawal Impairs mGluR5-Dependent Long-Term Depression in Nucleus Accumbens Shell Neurons of Both Direct and Indirect Pathways.
Huang CC, Liang YC, Lee CC, Hsu KS. Huang CC, et al. Mol Neurobiol. 2015 Dec;52(3):1223-1233. doi: 10.1007/s12035-014-8926-z. Epub 2014 Oct 16. Mol Neurobiol. 2015. PMID: 25319571 - Metabotropic glutamate receptor-mediated long-term depression: molecular mechanisms.
Gladding CM, Fitzjohn SM, Molnár E. Gladding CM, et al. Pharmacol Rev. 2009 Dec;61(4):395-412. doi: 10.1124/pr.109.001735. Epub 2009 Nov 19. Pharmacol Rev. 2009. PMID: 19926678 Free PMC article. Review.
Cited by
- Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why?
Wolf ME, Tseng KY. Wolf ME, et al. Front Mol Neurosci. 2012 Jun 27;5:72. doi: 10.3389/fnmol.2012.00072. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22754497 Free PMC article. - Adolescent alcohol exposure produces sex differences in negative affect-like behavior and group I mGluR BNST plasticity.
Kasten CR, Carzoli KL, Sharfman NM, Henderson T, Holmgren EB, Lerner MR, Miller MC, Wills TA. Kasten CR, et al. Neuropsychopharmacology. 2020 Jul;45(8):1306-1315. doi: 10.1038/s41386-020-0670-7. Epub 2020 Apr 8. Neuropsychopharmacology. 2020. PMID: 32268346 Free PMC article. - Cocaine and Amphetamine Induce Overlapping but Distinct Patterns of AMPAR Plasticity in Nucleus Accumbens Medium Spiny Neurons.
Jedynak J, Hearing M, Ingebretson A, Ebner SR, Kelly M, Fischer RA, Kourrich S, Thomas MJ. Jedynak J, et al. Neuropsychopharmacology. 2016 Jan;41(2):464-76. doi: 10.1038/npp.2015.168. Epub 2015 Jun 12. Neuropsychopharmacology. 2016. PMID: 26068728 Free PMC article. - Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies.
Joffe ME, Centanni SW, Jaramillo AA, Winder DG, Conn PJ. Joffe ME, et al. ACS Chem Neurosci. 2018 Sep 19;9(9):2188-2204. doi: 10.1021/acschemneuro.8b00200. Epub 2018 Jun 8. ACS Chem Neurosci. 2018. PMID: 29792024 Free PMC article. - Loss of mGluR1-LTD following cocaine exposure accumulates Ca2+-permeable AMPA receptors and facilitates synaptic potentiation in the prefrontal cortex.
Ruan H, Yao WD. Ruan H, et al. J Neurogenet. 2021 Sep-Dec;35(4):358-369. doi: 10.1080/01677063.2021.1931180. Epub 2021 Jun 7. J Neurogenet. 2021. PMID: 34092163 Free PMC article.
References
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. - PubMed
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 AA017037/AA/NIAAA NIH HHS/United States
- AA13641/AA/NIAAA NIH HHS/United States
- U01 AA013641/AA/NIAAA NIH HHS/United States
- DA19112/DA/NIDA NIH HHS/United States
- U24 AA013641/AA/NIAAA NIH HHS/United States
- U01 AA015635/AA/NIAAA NIH HHS/United States
- R01 DA019112/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases