A drug-inducible system for direct reprogramming of human somatic cells to pluripotency - PubMed (original) (raw)
A drug-inducible system for direct reprogramming of human somatic cells to pluripotency
Dirk Hockemeyer et al. Cell Stem Cell. 2008.
Abstract
Current approaches to reprogram human somatic cells to pluripotent iPSCs utilize viral transduction of different combinations of transcription factors. These protocols are highly inefficient because only a small fraction of cells carry the appropriate number and stoichiometry of proviral insertions to initiate the reprogramming process. Here we have generated genetically homogeneous "secondary" somatic cells, which carry the reprogramming factors as defined doxycycline (DOX)-inducible transgenes. These cells were obtained by infecting fibroblasts with DOX-inducible lentiviruses, isolating "primary" iPSCs in the presence of the drug, and finally differentiating to "secondary" fibroblasts. When "secondary" fibroblast lines were cultured in the presence of DOX without further viral infection, up to 2% of the cells were reprogrammed to pluripotent "secondary" human iPSCs. This system will facilitate the characterization of the reprogramming process and provides a unique platform for genetic or chemical screens to enhance reprogramming or replace individual factors.
Figures
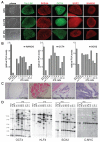
Figure 1. Characterization of DOX-inducible iPS cells derived from human fibroblasts
A. Phase contrast picture and immunofluorescence staining of human ES (hES) cells and iPS cell lines A6 and D1 for pluripotency markers SSEA4, Tra 1-60, OCT4, SOX2 and NANOG. B. Quantitative RT-PCR for the reactivation of the endogenous pluripotency related genes NANOG, OCT4 and SOX2 in independent iPS cell lines, hES cells and primary fibroblasts. Relative expression levels were normalized to the average expression of the two control hES cell lines. C. Hematoxilin and eosin staining of a teratoma sections generated from A1 iPS cells. D. Southern blot analysis of parental GM01660 fibroblasts, hES cells and iPS cells for proviral integrations of xbaI digested genomic DNA using 32P-labeld DNA probes against OCT4, KLF4, SOX2 and C-MYC.
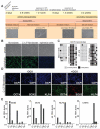
Figure 2. Characterization of genetically identical secondary fibroblasts derived from iPS cells
A. Timeline depicting the generation and reprogramming of secondary iPS cell-derived fibroblasts. B. Phase contrast picture and immunostaining of C1 secondary fibroblasts, primary fibroblasts and epithelial cells (EpRas) for the fibroblast marker prolyl-4-hydroxylase beta (P4H). C. Methylation analysis of the OCT4 promoter region. Light gray squares indicate unmethylated, and black squares indicate methylated CpGs in the OCT4 promoter of iPS cells, hES cells, primary, and secondary fibroblasts. D. Immunostaining for DOX-induced transgene expression of OCT4, KLF4 and SOX2 in C1 secondary fibroblasts 48 hours after drug administration. E. Quantitative RT-PCR for DOX-dependent transgene expression of OCT4, KLF4, SOX2 and C-MYC in secondary fibroblasts and primary infected fibroblasts. Relative expression levels were normalized to DOX-induced expression in primary infected fibroblasts.
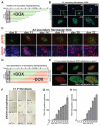
Figure 3. DOX-induced reprogramming of secondary fibroblast lines
A. Summary of reprogramming efficiency of in vitro differentiated and teratoma-derived secondary fibroblasts. Reprogramming efficiencies are categorized into “low”, indicating efficiency of< 0.1%, “medium”, 0.1–0.5%, and “high”, >0.5%. B. Phase contrast picture and immunofluorescence staining of secondary iPS cell line A6 for pluripotency markers SSEA4, Tra 1-60, OCT4, SOX2 and NANOG. C. Reprogramming efficiency of secondary fibroblasts A1, A2, C1 and D1 at 28 days (A1, A2) or 20 days (C1, D1) after DOX-induction as determined by immunostaining for Tra 1-60 and SSEA4. Efficiencies were calculated as the fraction of Tra 1-60/SSEA4 positive colonies to cells seeded. Error bars indicate the SEM generated from triplicates of the same experiment. D. Immunostaining of C1 secondary iPS colonies for Tra 1-60/SSEA4 and Tra 1-60/NANOG 20 days after DOX-induction.
Figure 4. Kinetics and requirement for transgene expression of secondary fibroblast reprogramming
A. Experimental outline of reprogramming kinetics experiment. Secondary fibroblasts were plated in DOX-free fibroblast medium on day -2 (black bars). After two days (day 0) medium was changed to hES medium containing DOX (green bars). Cells were fixed at the indicated time points and stained for pluripotency markers. B. Immunostaining of C1 secondary fibroblasts for Tra 1-60 at indicated days after DOX induction. Inset shows magnification of the same picture. C. Immunostaining of A2 secondary fibroblasts for Tra 1-60 and NANOG at the indicated days after DOX induction (upper panel). Lower panel shows a magnification of NANOG and DAPI staining of the same area. D. Experimental outline of the DOX withdrawal experiment. Secondary fibroblasts were plated in fibroblast medium without DOX on day -2 (black bars). After two days (day 0) medium was changed to human ES cell medium containing DOX (green bars). Medium was changed to hES medium without DOX at the indicated time points (red bars). Secondary iPS colonies were picked on day 20 for all DOX conditions. E. Immunostaining of secondary C1 iPS cells for SSEA4, Tra 1-60, OCT4 and NANOG, which were derived by DOX-induction for 8 days. F. Alkaline phosphatase staining of C1 secondary fibroblasts at day 20 after exposure to DOX. The day indicates the time when DOX was withdrawn from the culture medium. G. Number of reprogrammed colonies at different times after addition of DOX. The number of colonies on the indicated day was determined by immunostaining for Tra 1-60. Error bars indicate the SEM generated from triplicates of the same experiment. H. Number of reprogrammed colonies at day 20 after DOX addition. DOX was withdrawn from the culture medium at the indicated time points. The number of colonies was determined by immunostaining for Tra 1-60. Error bars indicate the SEM generated from triplicates of the same experiment.
Similar articles
- A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types.
Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. Wernig M, et al. Nat Biotechnol. 2008 Aug;26(8):916-24. doi: 10.1038/nbt1483. Epub 2008 Jul 1. Nat Biotechnol. 2008. PMID: 18594521 Free PMC article. - A high-efficiency system for the generation and study of human induced pluripotent stem cells.
Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K. Maherali N, et al. Cell Stem Cell. 2008 Sep 11;3(3):340-5. doi: 10.1016/j.stem.2008.08.003. Cell Stem Cell. 2008. PMID: 18786420 Free PMC article. - Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells.
Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R. Brambrink T, et al. Cell Stem Cell. 2008 Feb 7;2(2):151-9. doi: 10.1016/j.stem.2008.01.004. Cell Stem Cell. 2008. PMID: 18371436 Free PMC article. - Advances in reprogramming somatic cells to induced pluripotent stem cells.
Patel M, Yang S. Patel M, et al. Stem Cell Rev Rep. 2010 Sep;6(3):367-80. doi: 10.1007/s12015-010-9123-8. Stem Cell Rev Rep. 2010. PMID: 20336395 Free PMC article. Review. - Toward pluripotency by reprogramming: mechanisms and application.
Wang T, Warren ST, Jin P. Wang T, et al. Protein Cell. 2013 Nov;4(11):820-32. doi: 10.1007/s13238-013-3074-1. Protein Cell. 2013. PMID: 24078387 Free PMC article. Review.
Cited by
- Generation of induced pluripotent stem cells from human mesenchymal stem cells of parotid gland origin.
Yan X, Xu N, Meng C, Wang B, Yuan J, Wang C, Li Y. Yan X, et al. Am J Transl Res. 2016 Feb 15;8(2):419-32. eCollection 2016. Am J Transl Res. 2016. PMID: 27158336 Free PMC article. - Embryonic and induced pluripotent stem cells as a model for liver disease.
Yagi H, Tafaleng E, Nagaya M, Hansel MC, Strom SC, Fox IJ, Soto-Gutierrez A. Yagi H, et al. Crit Rev Biomed Eng. 2009;37(4-5):377-98. doi: 10.1615/critrevbiomedeng.v37.i4-5.40. Crit Rev Biomed Eng. 2009. PMID: 20528732 Free PMC article. Review. - Analysis of human and mouse reprogramming of somatic cells to induced pluripotent stem cells. What is in the plate?
Boué S, Paramonov I, Barrero MJ, Izpisúa Belmonte JC. Boué S, et al. PLoS One. 2010 Sep 17;5(9):e12664. doi: 10.1371/journal.pone.0012664. PLoS One. 2010. PMID: 20862250 Free PMC article. - Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells.
Mei Y, Saha K, Bogatyrev SR, Yang J, Hook AL, Kalcioglu ZI, Cho SW, Mitalipova M, Pyzocha N, Rojas F, Van Vliet KJ, Davies MC, Alexander MR, Langer R, Jaenisch R, Anderson DG. Mei Y, et al. Nat Mater. 2010 Sep;9(9):768-78. doi: 10.1038/nmat2812. Epub 2010 Aug 22. Nat Mater. 2010. PMID: 20729850 Free PMC article. - Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs.
Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Hanna J, et al. Proc Natl Acad Sci U S A. 2010 May 18;107(20):9222-7. doi: 10.1073/pnas.1004584107. Epub 2010 May 4. Proc Natl Acad Sci U S A. 2010. PMID: 20442331 Free PMC article.
References
- Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, Beard C, Brambrink T, Wu LC, Townes TM, Jaenisch R. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. - PubMed
- Lensch MW, Schlaeger TM, Zon LI, Daley GQ. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human-animal chimera. Cell Stem Cell. 2007;1:253–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HD045022/HD/NICHD NIH HHS/United States
- R37-CA084198/CA/NCI NIH HHS/United States
- R37 CA084198/CA/NCI NIH HHS/United States
- R01 HD045022/HD/NICHD NIH HHS/United States
- R01 CA084198/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials