The yeast lysosome-like vacuole: endpoint and crossroads - PubMed (original) (raw)
Review
The yeast lysosome-like vacuole: endpoint and crossroads
Sheena Claire Li et al. Biochim Biophys Acta. 2009 Apr.
Abstract
Fungal vacuoles are acidic organelles with degradative and storage capabilities that have many similarities to mammalian lysosomes and plant vacuoles. In the past several years, well-developed genetic, genomic, biochemical and cell biological tools in S. cerevisiae have provided fresh insights into vacuolar protein sorting, organelle acidification, ion homeostasis, autophagy, and stress-related functions of the vacuole, and these insights have often found parallels in mammalian lysosomes. This review provides a broad overview of the defining features and functions of S. cerevisiae vacuoles and compares these features to mammalian lysosomes. Recent research challenges the traditional view of vacuoles and lysosomes as simply the terminal compartment of biosynthetic and endocytic pathways (i.e. the "garbage dump" of the cell), and suggests instead that these compartments are unexpectedly dynamic and highly regulated.
Figures
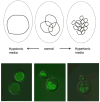
Figure 1. Vacuolar morphology is sensitive to growth conditions
The vacuole can change in volume and vesicle number depending on extracellular condtions. Top: Under normal conditions, the cell has two to three medium-sized vacuoles. In hypo-osmotic media, a single large vacuole takes up most of the cell. Upon hyper-osmotic shock, the vacuole fragments into multiple small lobes. Bottom: Superimposed images of fluorescently stained vacuoles on yeast cells viewed under Nomarski optics. Vacuoles were visualized using a C-terminally GFP-tagged V-ATPase subunit (Vma2p-GFP) during log-phase growth under hypotonic conditions (synthetic complete medium), normal conditions (YEPD, buffered to pH 5 (100 mM added salt)), and hypertonic conditions (YEPD, buffered to pH 5 with 1 M NaCl added).
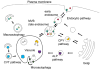
Figure 2. Trafficking Pathways to and from the Vacuole
Multiple vesicular pathways deliver proteins to and from the vacuole. Resident vacuolar proteins are sent to the vacuole by several biosynthetic pathways, while proteins targeted for degradaton may be sent via endocytosis or autophagy. Biosynthetic pathways: The ALP pathway delivers cargo from the late Golgi to the vacuole. The CPY pathway also starts from the late Golgi, but traverses multivesicular bodies (MVBs) before reaching the vacuole. The CVT pathway delivers biosynthetic cargo from the cytosol to the vacuole in a process that has common components with autophagy. Trafficking pathways away from the vacuole may recycle membrane and proteins to the endocytic or CPY pathways. Degradative pathways: Endocytosis transports both soluble and membrane-bound cargo from the plasma membrane and extracellular space to the vacuole. Cargo progresses from early endosomes to late endosomes (multivesicular bodies) before reaching the vacuole. In macroautophagy, vesicles called autophagosomes engulf cytosolic material for degradation at the vacuole. In microautophagy, the vacuolar membrane itself forms invaginations which form vesicles that are then degraded at the vacuole.
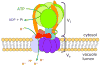
Figure 3. The Vacuolar H+-ATPase
The vacuolar H+-ATPase (V-ATPase) is a proton pump on the vacuolar membrane composed of a membrane-bound V0 sector and a cytosolic V1 sector. ATP hydrolysis in the V1 sector drives the translocation of protons through the V0 sector.
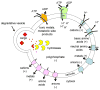
Figure 4. Constitutive Functions of The Yeast Vacuole
The vacuole is responsible for multiple constitutive processes: 1) degradation, 2) storage, 3) buffering, and 4) detoxification. 1) Degradation: The vacuole is highly enriched for hydrolases (yellow circles) that break down cargo delivered via multiple trafficking pathways to the vacuole. 2) Storage: The vacuolar membrane contains multiple transporters (blue polygons) that import amino acids, ions, and metals. Vacuolar acidification by the vacuolar H+ ATPase (V1 and V0, green) is required for proper maturation of hydrolases and for establishing the proton gradient that drives many transporters. 3) Buffering: The vacuolar membrane also contains transporters that recycle amino acids, ions, and metals to the cytosol. The combined activity of importers and exporters is important for ion homeostasis and amino acid recycling. The vacuole is also the main storage site for polyphosphate which buffers cations and is a source for cellular phosphate, 4) Detoxification: The vacuole also sequesters toxic metals and potentially harmful metabolic bi-products via ABC transporters.
Figure 5. Stress responses
Panel A: Upon nutrient deprivation, multiple autophagic pathways deliver specific and non-specific cargo to the vacuole. Macroautophagy is the non-specific degradation of cytosolic proteins and organelles. Autophagic membranes originate from pre-autosomal structures (PAS) that enclose cytosolic cargo, forming autophagosomes, which are then delivered to the vacuole. In microautophagy, vacuolar membranes invaginate and directly engulf cargo for degradation. Piecemeal microautophagy of the nucleus (PMN) is a type of autophagy that specifically degrades nuclear compartments, while pexophagy degrades peroxisomes via two mechanisms similar to macroautophagy and microautophagy. Panel B: Upon salt stress, vacuoles fragment into multiple tiny vesicles. This requires increased production of PI3,5P2 by the Fab1p lipid kinase. Calcium is released from vacuolar stores by Yvc1p, activating calcineurin and the Crz1p transcription factor. This leads to the increased production of the Ena1p Na+/Li+ pump at the plasma membrane and the Pho89p Na+/Pi+ symporter at the vacuole.
Similar articles
- The Fab1/PIKfyve phosphoinositide phosphate kinase is not necessary to maintain the pH of lysosomes and of the yeast vacuole.
Ho CY, Choy CH, Wattson CA, Johnson DE, Botelho RJ. Ho CY, et al. J Biol Chem. 2015 Apr 10;290(15):9919-28. doi: 10.1074/jbc.M114.613984. Epub 2015 Feb 20. J Biol Chem. 2015. PMID: 25713145 Free PMC article. - Yeast vacuoles: more than a model lysosome.
Armstrong J. Armstrong J. Trends Cell Biol. 2010 Oct;20(10):580-5. doi: 10.1016/j.tcb.2010.06.010. Epub 2010 Aug 3. Trends Cell Biol. 2010. PMID: 20685121 Review. - The vacuolar shapes of ageing: From function to morphology.
Aufschnaiter A, Büttner S. Aufschnaiter A, et al. Biochim Biophys Acta Mol Cell Res. 2019 May;1866(5):957-970. doi: 10.1016/j.bbamcr.2019.02.011. Epub 2019 Feb 21. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30796938 Review. - Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole.
Bryant NJ, Stevens TH. Bryant NJ, et al. Microbiol Mol Biol Rev. 1998 Mar;62(1):230-47. doi: 10.1128/MMBR.62.1.230-247.1998. Microbiol Mol Biol Rev. 1998. PMID: 9529893 Free PMC article. Review. - A lysosomal biogenesis map reveals the cargo spectrum of yeast vacuolar protein targeting pathways.
Eising S, Esch B, Wälte M, Vargas Duarte P, Walter S, Ungermann C, Bohnert M, Fröhlich F. Eising S, et al. J Cell Biol. 2022 Apr 4;221(4):e202107148. doi: 10.1083/jcb.202107148. Epub 2022 Feb 17. J Cell Biol. 2022. PMID: 35175277 Free PMC article.
Cited by
- The molecular components of the extracellular protein-degradation pathways of the ectomycorrhizal fungus Paxillus involutus.
Shah F, Rineau F, Canbäck B, Johansson T, Tunlid A. Shah F, et al. New Phytol. 2013 Nov;200(3):875-887. doi: 10.1111/nph.12425. Epub 2013 Jul 31. New Phytol. 2013. PMID: 23902518 Free PMC article. - The Lysosome at the Intersection of Cellular Growth and Destruction.
Shin HR, Zoncu R. Shin HR, et al. Dev Cell. 2020 Jul 20;54(2):226-238. doi: 10.1016/j.devcel.2020.06.010. Epub 2020 Jun 30. Dev Cell. 2020. PMID: 32610045 Free PMC article. Review. - Cloning of the Zygosaccharomyces bailii GAS1 homologue and effect of cell wall engineering on protein secretory phenotype.
Passolunghi S, Riboldi L, Dato L, Porro D, Branduardi P. Passolunghi S, et al. Microb Cell Fact. 2010 Jan 26;9:7. doi: 10.1186/1475-2859-9-7. Microb Cell Fact. 2010. PMID: 20102600 Free PMC article. - Genomic Characterization of the Titan-like Cell Producing Naganishia tulchinskyi, the First Novel Eukaryote Isolated from the International Space Station.
Bijlani S, Parker C, Singh NK, Sierra MA, Foox J, Wang CCC, Mason CE, Venkateswaran K. Bijlani S, et al. J Fungi (Basel). 2022 Feb 8;8(2):165. doi: 10.3390/jof8020165. J Fungi (Basel). 2022. PMID: 35205919 Free PMC article. - Impaired GCR1 transcription resulted in defective inositol levels, vacuolar structure and autophagy in Saccharomyces cerevisiae.
Ravi C, Gowsalya R, Nachiappan V. Ravi C, et al. Curr Genet. 2019 Aug;65(4):995-1014. doi: 10.1007/s00294-019-00954-2. Epub 2019 Mar 16. Curr Genet. 2019. PMID: 30879088
References
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. - PMC - PubMed
- Huh WK, Falvo JV, Gerke LC, Carroll AS, Howson RW, Weissman JS, O’Shea EK. Global analysis of protein localization in budding yeast. Nature. 2003;425:686–91. - PubMed
- Sarry JE, Chen S, Collum RP, Liang S, Peng M, Lang A, Naumann B, Dzierszinski F, Yuan CX, Hippler M, Rea PA. Analysis of the vacuolar luminal proteome of Saccharomyces cerevisiae. Febs J. 2007;274:4287–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous