A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice - PubMed (original) (raw)
A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice
Tobias Eckle et al. J Clin Invest. 2008 Oct.
Abstract
Although acute lung injury contributes significantly to critical illness, resolution often occurs spontaneously via activation of incompletely understood pathways. We recently found that mechanical ventilation of mice increases the level of pulmonary adenosine, and that mice deficient for extracellular adenosine generation show increased pulmonary edema and inflammation after ventilator-induced lung injury (VILI). Here, we profiled the response to VILI in mice with genetic deletions of each of the 4 adenosine receptors (ARs) and found that deletion of the A2BAR gene was specifically associated with reduced survival time and increased pulmonary albumin leakage after injury. In WT mice, treatment with an A2BAR-selective antagonist resulted in enhanced pulmonary inflammation, edema, and attenuated gas exchange, while an A2BAR agonist attenuated VILI. In bone marrow-chimeric A2BAR mice, although the pulmonary inflammatory response involved A2BAR signaling from bone marrow-derived cells, A2BARs located on the lung tissue attenuated VILI-induced albumin leakage and pulmonary edema. Furthermore, measurement of alveolar fluid clearance (AFC) demonstrated that A2BAR signaling enhanced amiloride-sensitive fluid transport and elevation of pulmonary cAMP levels following VILI, suggesting that A2BAR agonist treatment protects by drying out the lungs. Similar enhancement of pulmonary cAMP and AFC were also observed after beta-adrenergic stimulation, a pathway known to promote AFC. Taken together, these studies reveal a role for A2BAR signaling in attenuating VILI and implicate this receptor as a potential therapeutic target during acute lung injury.
Figures
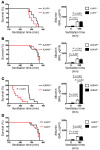
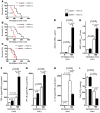
Figure 1. VILI in mice gene-targeted for individual ARs.
Previously characterized A1AR–/– (A; ref. 29), A2AAR–/– (B; ref. 30), A2BAR–/– (C), or A3AR–/– mice (D; ref. 22) or corresponding littermate controls were exposed to VILI, and survival times were determined during VILI. Mechanical ventilation was applied using pressure-controlled settings (inspiratory pressure of 35 mbar, inspired oxygen concentration 100%; respiratory rate and inspiratory/expiratory ratio were adjusted to maintain normal pH) until a cardiac standstill was observed in the surface electrocardiogram. Note the significantly attenuated survival of A2BAR–/– mice (C; P < 0.001, n = 8). Albumin concentration in the BAL fluid was determined by ELISA. For this purpose, the mice were mechanically ventilated using pressure-controlled ventilation with an inspired oxygen concentration of 100% for 180 minutes at 45 mbar. Note the significantly increased albumin concentration in the BAL fluid of A2BAR–/– mice (C; P < 0.001, n = 6).
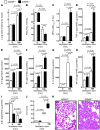
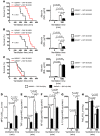
Figure 2. VILI in mice gene-targeted for the A2BAR.
(A–H) A2BAR–/– mice or littermate controls (A2BAR+/+) were mechanically ventilated using pressure-controlled ventilation with an inspired oxygen concentration of 100% over 180 minutes at 45 mbar. (A) Following ventilation, lungs were excised en bloc and weighed. Lungs were lyophilized for 48 hours, and lung water content (mg lung water/mg dry tissue) was determined. Results are presented as mean ± SD (n = 6). (B) To assess pulmonary gas exchange, blood gas analyses were performed by obtaining arterial blood via cardiac puncture. Analysis was performed immediately, and the ratio of the arterial partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) was determined. Results are presented as mean ± SD (n = 6). (C) Pulmonary neutrophil accumulation was quantified using a MPO assay. MPO activity was assessed using a spectrophotometric reaction with _O_-dianisidine hydrochloride. Absorbance at 450 nm was measured and reported as difference in OD (ΔOD) over 5 minutes. Results are presented as mean ± SD (n = 6). (D–I) TNF-α, IL-6, KC, IL-10, NF-κB, and IκBα levels were evaluated in lung tissue homogenates using a mouse ELISA. Results are presented as mean ± SD (n = 6). (J) For quantification of histological tissue damage by VILI following 180 min ventilation, VILI scores were assessed in A2BAR–/– or corresponding littermate control mice. Results are displayed as median (midline within boxes) and range (bars above and below boxes) (n = 4). (K) One of 4 representative photomicrographs (original magnification, ×200) stained with hematoxylin and eosin is displayed.
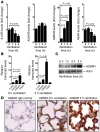
Figure 3. Transcriptional consequences of mechanical ventilation on AR expression.
(A) C57BL/6 mice were mechanically ventilated (inspiratory pressure of 35 mbar, 100% oxygen). After the indicated time periods, lungs were harvested, total RNA was isolated, and A1AR, A2AAR, A2BAR, and A3AR mRNA levels were determined by real-time RT-PCR. Data were calculated relative to the internal housekeeping gene (β-actin) and are expressed as mean fold change compared with control (0 min ventilation) ± SD at each indicated time (n = 4). Note selective induction of the A2BAR gene during high-pressure ventilation (10-fold, P < 0.01; n = 4). (B) Comparative gene expression of pulmonary ARs using real-time PCR. Relative expression levels in untreated controls or in mice after 180 min mechanical ventilation (inspiratory pressure of 35 mbar, 100% oxygen) are shown. Values are expressed as mean ± SEM (n = 4). *P < 0.05 compared with A2AAR. (C) Mice were mechanically ventilated (35 mbar inspiratory pressure, 100% oxygen), and lungs were harvested at the indicated time points, shock frozen, and lysed, and proteins were resolved by SDS-PAGE. Resultant western blots were probed with anti-A2BAR antibodies. To control for loading conditions, blots were stripped and reprobed for actin expression. One representative experiment of 3 is shown. (D)
To examine the influence of mechanical ventilation on pulmonary A2BAR expression patterns, C57BL/6 mice were ventilated in a pressure-controlled setting over 0 h or 3 h (35 mbar inspiratory pressure, 100% inspired oxygen concentration). Lungs were stained with antibodies for A2BAR. IgG controls were used at identical concentrations and staining conditions as the target primary antibodies (original magnification, ×400; n = 4).
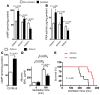
Figure 4. cAMP levels and PKA activity during VILI.
(A and B) To assess cAMP levels and PKA activity in pulmonary tissues during VILI, A2BAR–/– mice and littermate controls were mechanically ventilated using pressure-controlled ventilation with an inspired oxygen concentration of 100% over 180 minutes at 45 mbar. Animals were euthanized, and lungs were perfused with 5 ml of PBS through the right ventricle. Lungs were excised, shock-frozen utilizing liquid nitrogen, and mechanically homogenized. cAMP levels (A) and PKA activity (B) were determined by ELISA. Note that the increases in cAMP and PKA activity associated with mechanical ventilation were abolished in A2BAR–/– mice. (C) Treatment with the specific β2-adrenergic agonist zinterol during VILI. Control C57BL/6 mice were treated with 300 μl 10–7 M intratracheal zinterol, and baseline cAMP levels were determined in lung homogenates using a competitive immunoassay kit. (D) Control C57BL/6 mice were treated with 300 μl 10–7 M intratracheal zinterol or vehicle control, followed by mechanical ventilation using pressure-controlled settings at an inspired oxygen concentration of 100% and 45 mbar inspiratory pressure for 0 or 180 min. Albumin concentration in the BAL fluid was determined by murine ELISA. (E) Survival times during VILI after 300 μl 10–7 M intratracheal zinterol or vehicle treatment. Mechanical ventilation was applied using pressure-controlled settings (inspiratory pressure of 35 mbar, inspired oxygen concentration 100%, respiratory rate and inspiratory/expiratory ratio were adjusted to maintain normal pH) until a cardiac standstill was observed in the surface electrocardiogram.
Figure 5. A2BAR antagonist treatment PSB1115 during VILI.
(A–C), A2BAR+/+, A2AAR–/–, and A2BAR–/– mice and their corresponding littermate controls were treated with 10 mg/kg PSB1115 or vehicle 30 minutes prior to induction of anesthesia. Mechanical ventilation was begun, and mice were ventilated using pressure-controlled settings (inspiratory pressure of 45 mbar, 100% inspired oxygen concentration) until a cardiac standstill was observed in the surface electrocardiogram
(P < 0.01, n = 8). (D) Mechanical ventilation was begun, and mice were ventilated for 0 or 180 minutes using pressure-controlled settings (inspiratory pressure of 45 mbar, 100% inspired oxygen concentration). Albumin concentration in the BAL fluid was determined by ELISA (n = 6). (E) Pulmonary neutrophil sequestration was quantified using a MPO assay. MPO activity was assessed using a spectrophotometric reaction with _O_-dianisidine hydrochloride. Absorbance at 450 nm was measured and reported as difference in OD over 5 minutes (n = 6). (F–H) TNF-α, NF-κB, and IL-10 levels were evaluated in lung tissue homogenates using a murine ELISA (n = 6). (I) To assess pulmonary gas exchange, blood gas analyses were performed by obtaining arterial blood via cardiac puncture. Analysis was performed immediately, and the ratio of the arterial partial pressure of oxygen to the fraction of inspired oxygen was determined. Results are presented as mean ± SD (n = 6).
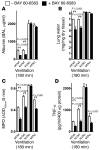
Figure 6. A2BAR agonist (BAY 60-6583) treatment.
(A–C) A2BAR+/+, A2AAR–/–, and A2BAR–/– mice and their corresponding littermate controls were treated with 2 mg/kg BAY 60-6583 or vehicle 30 minutes prior to induction of anesthesia. Mechanical ventilation was begun, and mice were ventilated using pressure-controlled settings (inspiratory pressure of 45 mbar, 100% inspired oxygen concentration) until a cardiac standstill was observed in the surface electrocardiogram (P < 0.01, n = 8). In other studies, albumin concentrations in the BAL fluid were determined by ELISA after mechanical ventilation using pressure-controlled settings with an inspired oxygen concentration of 100% for 180 minutes at 45 mbar. (D) Pulmonary neutrophil sequestration was quantified using a MPO assay (n = 6). (E–G) TNF-α, NF-κB, and IL-10 levels were evaluated in lung tissue homogenates using murine ELISA (n = 6). (H) To assess pulmonary gas exchange, blood gas analyses were performed by obtaining arterial blood via cardiac puncture. The ratio of the arterial partial pressure of oxygen to the fraction of inspired oxygen was determined. Results are presented as mean ± SD (n = 6).
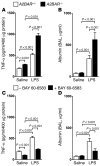
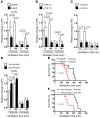
Figure 7. A2BAR signaling during LPS-induced lung injury.
(A and B) A2BAR–/– and A2BAR+/+ mice were exposed to LPS inhalation for 30 minutes. Twenty-four hours after LPS exposure, TNF-α in lung tissue homogenates and albumin concentration in the BAL fluid were determined using a murine ELISA (n = 6). (C and D) A2BAR+/+ mice received 2 mg/kg BAY 60-6583 i.p. or were treated with vehicle 30 minutes prior to LPS inhalation. (C) TNF-α levels in lung tissues and (D) albumin concentrations in the BAL fluid (n = 6).
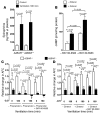
Figure 8. VILI in A2BAR bone marrow–chimeric mice.
A2BAR bone marrow–chimeric mice were subjected to VILI using mechanical ventilation for 180 minutes at an inspiratory pressure of 45 mbar and 100% inspired oxygen concentration. (A) Albumin concentration in the BAL fluid was determined by ELISA. WT/WT, A2BAR+/+/A2BAR+/+; KO/WT, A2BAR–/–/A2BAR+/+;
KO/KO, A2BAR–/–/ A2BAR–/–; WT/KO, A2BAR+/+/A2BAR–/–. (B) Following ventilation at the indicated settings, lungs were excised en bloc and weighed. Lungs were lyophilized for 48 hours, and lung water content (mg lung water/mg dry tissue) was determined. Note the increased albumin concentration and lung water content in A2BAR–/–/A2BAR–/– and A2BAR+/+/A2BAR–/– mice compared with A2BAR+/+/A2BAR+/+ mice (P < 0.001). (C) Pulmonary neutrophil sequestration was quantified using a MPO assay. MPO activity was assessed using a spectrophotometric reaction with _O_-dianisidine hydrochloride. Absorbance at 450 nm was measured and reported as difference in OD over 5 minutes. (D–F) TNF-α, IL-6, and KC levels were evaluated in lung tissue homogenates using a mouse ELISA. Note the similar degree of pulmonary inflammation in A2BAR–/–/A2BAR+/+ and A2BAR+/+/A2BAR–/– mice compared with A2BAR+/+/A2BAR+/+ mice. n = 6.
Figure 9. BAY 60-6583–dependent lung protection during VILI in A2BAR bone marrow–chimeric mice.
(A–D) A2BAR bone marrow–chimeric mice were treated with BAY 60-6583 (2 mg/kg) 30 minutes prior to induction of anesthesia. Mechanical ventilation was begun, and mice were ventilated using an inspiratory pressure of 45 mbar, 100% inspired oxygen concentration, for 180 minutes. Results are presented as mean ± SD. n = 6. (A) Albumin concentration in the BAL fluid was determined by ELISA. (B) Following mechanical ventilation, lungs were excised en bloc and weighed. Lungs were then lyophilized for 48 hours, and lung water content (mg lung water/mg dry tissue) was determined. Capillary-alveolar barrier protection was observed only in A2BAR+/+/A2BAR+/+ and A2BAR–/–/A2BAR+/+ mice (P < 0.001; n = 6). (C) Pulmonary neutrophil sequestration was quantified using a MPO assay. MPO activity was assessed using a spectrophotometric reaction with _O_-dianisidine hydrochloride. Absorbance at 450 nm was measured and reported as difference in OD over 5 minutes. (D) TNF-α levels were evaluated in lung tissue homogenates using a mouse ELISA. Note the similar levels of pulmonary inflammation in A2BAR–/–/A2BAR+/+ and A2BAR+/+/A2BAR–/– mice.
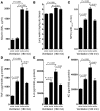
Figure 10. Contribution of A2BAR signaling to AFC during VILI.
(A) To determine whether A2BAR signaling affects pulmonary fluid transport, we measured AFC using a mechanically ventilated live mouse model. A2BAR–/– mice and littermate controls were mechanically ventilated in a pressure-controlled setting at 45 mbar for 0 to 180 minutes. AFC was measured by instilling 300 μl of iso-osmolar 0.9% NaCl solution with 5% BSA. Mechanical ventilation was continued for 30 minutes, and AFC was measured in the presence or absence of the ENaC inhibitor amiloride (1 mM). *P < 0.01 compared with no amiloride. n = 8. (B) Influence of A2BAR antagonist PSB1115 on AFC during VILI. Following induction of VILI, control mice received PSB1115 (1 μM) alone or in combination with amiloride (1 mM), and AFC was determined. *P < 0.01 compared with no amiloride. n = 8. (C) VILI was induced in A2BAR bone marrow–chimeric mice, and AFC was determined. *P < 0.001 compared with A2BAR+/+/A2BAR+/+. n = 8. (D) Influence of A2BAR agonist BAY 60-6583 on AFC during VILI. Following induction of VILI, control mice received BAY 60-6583 (1 μM) alone or in combination with amiloride (1 mM), and AFC was determined *P < 0.01 compared with no amiloride. n = 8. (E and F) Control mice were treated with intratracheal BAY 60-6583 (1 μM, 100 μl) and/or amiloride (1 mM) or vehicle following tracheotomy and initiation of mechanical ventilation. Mice were ventilated using pressure-controlled settings (inspiratory pressure of 45 mbar, 100% inspired oxygen concentration) until a cardiac standstill was observed in the surface electrocardiogram. Note that the longer survival time during VILI with A2BAR agonist treatment was abolished following amiloride treatment (n = 8).
Figure 11. Influence of β2-adrenergic and/or A2BAR signaling on AFC during VILI.
(A) Epinephrine plasma levels in A2BAR–/– and A2BAR+/+ mice that were mechanically ventilated in a pressure-controlled setting at 45 mbar over 180 minutes. (B) Basal cAMP levels in lung tissue from A2BAR+/+ mice that were treated with zinterol and/or BAY 60-6583. (C and D) To determine β2-adrenergic and A2BAR signaling effects on pulmonary fluid transport, A2BAR–/– and A2BAR+/+ mice were mechanically ventilated in a pressure-controlled setting at 45 mbar for 0 to 180 minutes. AFC was measured by instilling 300 μl of iso-osmolar 0.9% NaCl solution with 5% BSA. Mechanical ventilation was continued for 30 minutes, and AFC was measured in the presence or absence of the nonselective β-adrenergic receptor antagonist propranolol (intratracheal instillation of 10–4 M propranolol combined with 3 mg/kg i.p.) with or without BAY 60-6583 (10–3 M to the instilled fluid) or in the presence or absence of the β-adrenergic agonist zinterol (intratracheal, 10–7 M). *P < 0.01 compared with no propranolol (C) or zinterol (D), by ANOVA with Bonferroni post-hoc test. n = 8. In subsets of experiments, either propranolol or zinterol were added together with BAY 60-6583 10–3 M to the instilled fluid. §P < 0.01 compared with propranolol alone (C) or zinterol alone (D), by ANOVA with Bonferroni post-hoc test. n = 8.
Similar articles
- Adenosine A2B receptor activation stimulates alveolar fluid clearance through alveolar epithelial sodium channel via cAMP pathway in endotoxin-induced lung injury.
Wang M, Guo X, Zhao H, Lv J, Wang H, An Y. Wang M, et al. Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L787-L800. doi: 10.1152/ajplung.00195.2019. Epub 2020 Mar 4. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32129084 - Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury.
Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Schingnitz U, et al. J Immunol. 2010 May 1;184(9):5271-9. doi: 10.4049/jimmunol.0903035. Epub 2010 Mar 26. J Immunol. 2010. PMID: 20348420 Free PMC article. - Adenosine receptors and diabetes: Focus on the A(2B) adenosine receptor subtype.
Merighi S, Borea PA, Gessi S. Merighi S, et al. Pharmacol Res. 2015 Sep;99:229-36. doi: 10.1016/j.phrs.2015.06.015. Epub 2015 Jul 2. Pharmacol Res. 2015. PMID: 26142494 Review. - The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases.
Eisenstein A, Patterson S, Ravid K. Eisenstein A, et al. J Cell Physiol. 2015 Dec;230(12):2891-7. doi: 10.1002/jcp.25043. J Cell Physiol. 2015. PMID: 25975415 Free PMC article. Review.
Cited by
- Therapeutic targeting of hypoxia inducible factor in acute respiratory distress syndrome.
Tran TT, Eltzschig HK, Yuan X. Tran TT, et al. J Physiol. 2024 Nov;602(21):5745-5756. doi: 10.1113/JP284599. Epub 2023 Nov 30. J Physiol. 2024. PMID: 38031820 Review. - The inflammatory tissue microenvironment in IBD.
Colgan SP, Curtis VF, Campbell EL. Colgan SP, et al. Inflamm Bowel Dis. 2013 Sep;19(10):2238-44. doi: 10.1097/MIB.0b013e31828dcaaf. Inflamm Bowel Dis. 2013. PMID: 23702808 Free PMC article. Review. - Adenosine A2B Receptor: From Cell Biology to Human Diseases.
Sun Y, Huang P. Sun Y, et al. Front Chem. 2016 Aug 24;4:37. doi: 10.3389/fchem.2016.00037. eCollection 2016. Front Chem. 2016. PMID: 27606311 Free PMC article. Review. - Human Neutrophil Defensins Disrupt Liver Interendothelial Junctions and Aggravate Sepsis.
Chen Q, Yang Y, Pan Y, Shen L, Zhang Y, Zheng F, Shu Q, Fang X. Chen Q, et al. Mediators Inflamm. 2022 Jul 29;2022:7659282. doi: 10.1155/2022/7659282. eCollection 2022. Mediators Inflamm. 2022. PMID: 35935811 Free PMC article. - The adenosinergic immunomodulatory drugs.
Ohta A, Sitkovsky M. Ohta A, et al. Curr Opin Pharmacol. 2009 Aug;9(4):501-6. doi: 10.1016/j.coph.2009.05.005. Epub 2009 Jun 17. Curr Opin Pharmacol. 2009. PMID: 19539527 Free PMC article. Review.
References
- Reutershan J., Cagnina R.E., Chang D., Linden J., Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J. Immunol. 2007;179:1254–1263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases